It is expected that obstetricians–gynecologists, primary care providers, and internists be able to evaluate and treat the majority of couples with infertility. Infertility specialists may be necessary in complex cases, when first-line therapy is unsuccessful, or when assisted reproductive technologies are needed. An overview of the diagnosis and treatment of infertility is provided. The imaging modalities helpful for infertility evaluation, within the context of the overall care of the infertile couple, are presented.
Disease
Definition
Infertility is defined as the failure to conceive after 1 year of unprotected intercourse.
Prevalence and Epidemiology
Infertility affects approximately one in six couples in the United States. The latest data on infertility available are from the 2002 National Survey of Family Growth. Of approximately 60 million women of reproductive age in 2002, 12% (>7 million) had received infertility services at some point in their lives. Additionally, 7.4% of married couples in which the woman was of reproductive age reported that they had not used contraception for 12 months and the women had not become pregnant.
In the past decade, the demand for infertility services has escalated, and is expected to increase as more women enter the work force and delay childbearing. Although it may appear that the incidence of infertility is increasing, it has remained relatively stable during the past 3 decades. The apparent discrepancy results from the large increase in infertility services offered to women between the ages of 35 and 44, of whom approximately 35% to 40% will experience a reduced fertility potential. Unfortunately, less than half of infertile couples seek care, primarily because infertility is often not perceived as a legitimate health problem by third-party payers. Currently only a handful of states mandate coverage for infertility; therefore the financial burden of services often falls to the infertile couple.
Etiology and Pathophysiology
In approximately 40% to 50% of cases of infertility, a female factor is identified. The most common causes of infertility are listed in Box 16-1 .
- ▪
Tubal factor
- ▪
Ovulatory dysfunction
- ▪
Diminished ovarian reserve
- ▪
Endometriosis
- ▪
Uterine factor
- ▪
Male factor
- ▪
Other factor
- ▪
Unknown factor
- ▪
Multiple factors
- •
Female factors only
- •
Female and male factors
- •
Gonadotropin-releasing hormone (GnRH) secreted by the hypothalamus stimulates follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion from the anterior pituitary gland. FSH stimulates folliculogenesis and estradiol production by the ovary. The estradiol production feeds back negatively on the hypothalamus and pituitary glands, resulting in the suppression of FSH and LH concentrations. The increasing estradiol concentration during the follicular phase of the menstrual cycle results in proliferation of the uterine endometrium.
The dominant follicle increases in size to approximately 20 to 26 mm. At midcycle, there is a switch from negative feedback control of LH secretion by ovarian hormones (estrogen and progesterone) to a positive feedback effect, resulting in an LH surge. Ovulation occurs approximately 36 hours after the onset of the LH surge. The oocyte is released and travels down the fallopian tube to the uterine cavity.
The granulosa cells surrounding the oocyte, under the influence of LH, secrete progesterone during the luteal phase. Increasing progesterone levels result in endometrial changes (secretory pattern). In the absence of fertilization, estrogen and progesterone levels decrease, resulting in endometrial sloughing and the onset of menses approximately 14 days after the LH surge. In response to falling corpus luteum steroid production, the hypothalamic–pituitary gland is released from negative feedback and FSH levels increase, thereby beginning a new cycle. If the oocyte becomes fertilized, it implants in the endometrium and begins to secrete chorionic gonadotropin, which maintains the corpus luteum and progesterone production during the first 8 to 10 weeks of pregnancy.
Ovulatory Disorders
Ovulatory disorders are present in approximately 25% of infertile women. The most common ovulation disorder is polycystic ovary syndrome (PCOS). The first-line therapy for ovulation disorder is usually ovulation induction with clomiphene citrate (CC). Approximately 80% of women with PCOS will respond to CC. In patients in whom CC is unsuccessful, alternatives for ovulation induction include injectable gonadotropins.
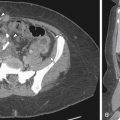
Hyperprolactinemia is another endocrine cause of ovulatory dysfunction and is most often the result of a pituitary adenoma ( Figure 16-1 ). Hyperprolactinemia can result in hypogonadotropic hypogonadism and is usually successfully treated with a dopamine agonist such as bromocriptine.

Premature ovarian failure is defined as menopause before age 40. The only effective treatment is egg donation, which yields pregnancy rates of approximately 50% per cycle.
Advanced Maternal Age and Decreased Ovarian Reserve
Fertility declines with increasing maternal age, particularly after age 40. The probability of pregnancy is inversely proportional to the age of the female partner, with younger women (<30 years) having a higher probability of success. The incidence of chromosomal abnormalities and spontaneous abortion also increases with increased maternal age. The most recent Centers for Disease Control and Prevention (CDC) statistics (2007) reveal a live birth rate of just 11% among women aged 41 to 42, and 4% among women older than 42.
Cycle fecundability is defined as the probability that a single cycle will result in pregnancy, whereas cycle fecundity is defined as the probability that a single cycle will result in a live birth. Pregnancy rates decrease for all forms of infertility treatment strategies with advancing maternal age. Although multiple factors may play a role in the age-related decline in fertility, changes related to the aging oocyte are thought to be the predominant mechanisms in reduced fertility.
Fecundability estimates are used to evaluate a woman’s likelihood of achieving pregnancy. Procedures associated with low fecundability rates or poor outcomes are inadvisable in older women with a decreasing fertility potential. In women less than age 30, roughly one in four conceive in the first month of attempting pregnancy, and ultimately 80% conceive after 1 year of unprotected intercourse. Unfortunately the prospects for older women are not as favorable. Monthly fecundability estimates decrease with aging, with less than 60% of women over age 35 conceiving within the first year of attempting pregnancy. In women between the ages of 40 and 42, monthly fecundability estimates continue to decrease, ranging between 5% and 10%.
Tubal Disease
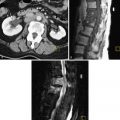
Traditionally tubopelvic disease was treated surgically ( Figure 16-2 ). Today in vitro fertilization (IVF) is the mainstay of treatment for tubal disease. Approximately 20% of infertile women have tubal disease. Tubal disease is usually diagnosed by hysterosalpingography (HSG) or laparoscopy. Proximal occlusion can sometimes be treated by fallopian tube catheterization under fluoroscopic guidance. Laparoscopic or microsurgical techniques can also be used to treat tubal disease with success rates ranging from 15% to 35%, depending on the extent of the adhesions. IVF, however, is the usual treatment for tubal infertility, with success rates of approximately 33% per cycle. The presence of hydrosalpinges, especially when large and bilateral, has been shown to decrease pregnancy rates with IVF. In such cases, bilateral salpingectomy before IVF has been shown to improve pregnancy rates.

Endometriosis
Endometriosis is found in 5% to 10% of infertile female partners. Endometriosis is associated with infertility, but a causal relationship, except in cases of moderate to severe disease, is unclear. Moderate or severe endometriosis may cause anatomic distortion, which may be responsible for infertility. Surgical laparoscopic treatment is associated with an improved pregnancy success rate.
With mild or minimal endometriosis, the causal relationship is less clear. Whether surgical treatment of minimal or mild endometriosis improves pregnancy rates is controversial. It is common practice to treat even mild or minimal disease at the time of diagnosis in cases of otherwise unexplained infertility.
IVF has also been shown to significantly increase pregnancy rates in patients with endometriosis. Implantation and pregnancy rates, however, remain lower than in women undergoing IVF for tubal disease, implying an endometrial defect and/or additional oocyte or embryo quality defect.
Causes of Male Infertility
Male infertility may be either congenital or acquired and can be classified as pretesticular, testicular, and posttesticular. In nearly 50% of infertile men it is not possible to identify a cause, and hence the infertility is defined as unexplained or idiopathic. The causes of male infertility are listed in Box 16-2 .
- ▪
Pretesticular
- •
Endocrinopathies
e.g., Kallman’s syndrome, hyperprolactinemia
- •
- ▪
Testicular
- •
Genetic
- •
Klinefelter’s syndrome
- •
Y chromosome microdeletions
- •
- •
Congenital
- •
Cryptorchidism
- •
- •
Varicocele
- •
Radiation
- •
Chemotherapy
- •
Drugs, e.g.,
- •
Torsion or trauma
- •
Infectious
- •
Idiopathic
- •
- ▪
Posttesticular
- •
Obstructive
- •
Congenital, e.g., congenital bilateral aplasia of vas deferens
- •
Acquired, e.g., vasectomy
- •
- •
Other
- •
Ejaculatory dysfunction
- •
Immunologic
- •
- •
In the male partner, a history of undescended testicles, previous problems with fertility, history of testicular trauma or infection, family history of infertility, cancer, hypertension, and diabetes may indicate a male factor. Sexual dysfunction may result from pituitary tumors or medical treatment of hypertension and vascular disease.
Oligospermia is defined as a sperm concentration less than 20 million/mL. Azoospermia is the complete absence of sperm in the ejaculate and occurs in 2% to 8% of infertile men. Azoospermia may be caused by obstruction of the extratesticular ductal system (obstructive azoospermia) or defects in spermatogenesis (nonobstructive azoospermia). In nonobstructive azoospermia, spermatogenesis is impaired, and this can be caused by primary testicular failure or secondary to hypothalamic–pituitary insufficiency. A testicular biopsy is required to distinguish between Sertoli cell–only syndrome, hypospermatogenesis, spermatogenic arrest, and obstructive forms.
Unexplained Infertility
In approximately 15% of infertile couples all tests are normal, and these cases are classified as unexplained infertility. It has been suggested that couples with unexplained infertility have a subtle and perhaps transient defect in reproductive capacity that cannot be easily appreciated with our currently available diagnostic methods. The treatment is generally empiric-controlled ovarian hyperstimulation and intrauterine insemination (IUI), the rationale being to increase the probability of pregnancy.
Manifestations of Disease
Clinical Presentation
Infertility Evaluation
Eighty-five percent of couples attempting conception will have been successful after 1 year of regular timed unprotected intercourse. Clinical evaluation of infertility is indicated if a pregnancy has not occurred by that time ( Box 16-3 ). Earlier assessment is warranted in women greater than 35 years of age or in women with known risk factors for infertility, such as oligoovulation or known pelvic pathology.
- ▪
History and physical examination
- ▪
Hysterosalpingography and/or laparoscopy
- ▪
Semen analysis
- ▪
Other, e.g., day 3 follicle-stimulating hormone test, hysteroscopy, pelvic ultrasound, sonohysterogram
Evaluation of the Infertile Female
A comprehensive infertility evaluation includes reviewing general health, risk factors for infertility, and performing a physical examination. The consultation needs to meet two general objectives: (1) The potential causes of infertility should be reviewed with the couple; and (2) the couple should leave with a plan that outlines future testing and treatment. Treatment plans are generally limited to a short interval because nearly 60% of couples conceive within the first 3 to 4 months of directed therapy. Longer treatment plans are often frustrating and unproductive.
Psychological needs also need to be recognized and addressed. Infertility may be one of the most stressful events for couples seeking infertility services. Support groups, such as RESOLVE, may be a tremendous help in limiting stress. At times, couples may need more personal guidance. A competent therapist can teach patients stress management strategies and coping and decision-making skills to facilitate their journey through complex treatment options. The American Society for Reproductive Medicine (ASRM) has guidelines for professional counselors in reproductive medicine, and many are members of the psychological special interest group of ASRM. It is now recognized that couples who have good coping and partner communication skills and can effectively manage stress have a better chance at conceiving.
A careful menstrual history is essential. A history of irregular menses may mean infrequent ovulation. Amenorrhea may be caused by an eating disorder, exercise, or a pituitary adenoma. Associated galactorrhea or hirsutism may support the diagnosis of other endocrine diseases that affect ovulation. Dysmenorrhea, pelvic pain, and/or dyspareunia may be associated with endometriosis or pelvic infection. A positive history of genital infection, particularly gonorrhea and chlamydia, intrauterine device use, or a history of ectopic pregnancy is associated with a higher risk for tubal disease. Pelvic or abdominal surgery is a recognized risk factor for pelvic adhesions and associated infertility.
Physical Examination
In the female partner, a careful pelvic examination is necessary to exclude pelvic tenderness, adnexal discomfort or masses, uterine retroversion and fixation, cervical motion tenderness, and uterosacral nodularity.
Ovarian Reserve Tests
The decline in fecundity associated with maternal age is caused by both a marked reduction in oocyte number and oocyte quality. During fetal development the fetal ovary contains approximately 6 to 7 million primordial follicles. Before birth a majority of these follicles undergo apoptosis, resulting in a follicular pool containing approximately 1 to 2 million oocytes. Follicular loss continues at a decreased rate throughout childhood, such that there are 300,000 to 400,000 oocytes present at menarche. By menopause the number of follicles decreases to less than 1000. The decline in oocyte quality is caused largely by an increasing rate of aneuploidy secondary to an increased rate of meiotic disjunction that correlates directly with maternal age.
The primary goal of any ovarian reserve test is to assess the quantity and quality of oocytes at any given maternal age. Although maternal age is a consistent marker that correlates with ovarian reserve, it is well recognized that diminished ovarian reserve can occur at any age. Both serum and ultrasound markers are now used clinically to characterize ovarian reserve and thereby predict reproductive potential.
FSH Testing
The easiest and most widely used measurement of ovarian reserve is the basal (cycle day 3) serum FSH. Generally a basal FSH level greater than 10 to 15 IU/L is considered abnormal. The numerous studies assessing the ability of the basal FSH to predict a successful birth after IVF are somewhat inconsistent. Recently a large systematic review concluded that the accuracy of the basal FSH in predicting a poor response to fertility medications is adequate, but the accuracy of predicting a live birth is only reliable if a high FSH threshold level is set. Furthermore, because of the variety of available FSH assay systems, results can vary significantly between different laboratories. In consideration of these issues, the basal FSH concentration is most suitable for use as a screening test, helping to guide further diagnostic evaluation.
Inhibin B
An additional potential serum marker for assessing ovarian reserve is Inhibin B. Both Inhibin A and Inhibin B are heterodimeric substances that are detectable in the peripheral circulation during the menstrual cycle. Inhibin A is predominantly produced by the corpus luteum, whereas Inhibin B is produced primarily by granulosa cells of the developing cohort of antral follicles. Inhibin B levels correlate with the pool of FSH-responsive follicles, but studies addressing the predictive value of Inhibin B in the ovarian response during IVF or pregnancy outcome have provided conflicting results. Moreover, because there is no accepted international standard for the Inhibin B assay, correlation between different laboratories is difficult.
Clomiphene Challenge Test
The most widely used of these tests is the clomiphene challenge test (CCCT), which involves administration of 100 mg of CC on days 5 to 9 of the menstrual cycle. FSH and estradiol are measured on day 3, and FSH is measured on day 10 of the cycle. A test is considered to be abnormal when either estradiol or FSH is elevated. The proposed benefit to the CCCT is that it may identify women with decreased ovarian reserve who might otherwise not be identified by basal FSH or estradiol alone. Despite initial studies, comparative studies have shown that the CCCT does not provide a significant advantage over the basal FSH concentration in the assessment of ovarian response. The clinical value of the CCCT is further limited by the increased labor, cost, and high false-positive rate associated with the test.
Antimullerian Hormone
Antimullerian hormone (AMH), also referred to as mullerian-inhibiting substance, is a putative marker of ovarian reserve. It is expressed by the granulosa cells of small ovarian follicles and reflects the pool of primordial follicles within the ovary. Women with a normal FSH demonstrate a gradual decrease in IVF success rates beginning after age 30. In contrast to FSH, AMH levels decrease after age 30, reflecting the potential advantage of this serum test as an early marker of declining ovarian reserve. AMH levels decrease as the total size of the primordial follicle pool decreases, and decrease to essentially zero with menopause. Compared with serum and ultrasound tests of ovarian reserve, AMH levels demonstrate the least intracycle variability, and values are consistent across the menstrual cycle. Levels greater than 0.7 ng/mL are associated with adequate ovarian reserve, but well-established cutoff levels in the prediction of pregnancy success or failure are currently lacking. Although studies evaluating the predictive capacity of AMH are promising, additional studies are needed to evaluate whether AMH offers a diagnostic advantage compared with other available tests.
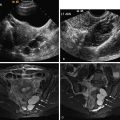
Antral Follicle Count by Ultrasound
Antral follicle count (AFC) and ovarian volume measures correlate with maternal age and the ovarian response during assisted reproductive technology (ART) procedures ( Figure 16-3 ). This is the only imaging method for estimating ovarian reserve, and is described in more detail in the section on Imaging Techniques and Findings.