This chapter describes the role of noninvasive imaging modalities in the assessment of vascular manifestations of inflammatory and infectious disorders. Indications, limitations, technical aspects, and pitfalls and pearls of the following noninvasive imaging modalities are discussed: ultrasonography and color-coded Duplex sonography (CCDS), computed tomography (CT), magnetic resonance imaging (MRI) and MR angiography (MRA), fluorine-18 deoxyglucose positron emission tomography (FDG PET), and digital subtraction angiography (DSA). The chapter is subdivided in the two major parts: inflammatory disorders and infectious disorders.
Inflammatory Disorders
Since the 1992 Chapel Hill, North Carolina consensus conference, primary systemic vasculitides have been subdivided according to the size of the affected vessel into large vessel vasculitis, medium vessel vasculitis, and small vessel vasculitis. Large vessel vasculitides include giant cell arteritis (GCA) and Takayasu arteritis (TA), whereas medium vessel vasculitides include polyarteritis nodosa (PAN) and Kawasaki disease. Small vessel vasculitides are further subdivided into those that demonstrate an association with antineutrophil cytoplasmic antibody (ANCA), such as granulomatosis with polyangitis formerly known as Wegener granulomatosis, Churg-Strauss vasculitis, and microscopic polyangiitis, and those small vessel vasculitides that are associated with immune complexes in the vessel. Inflammatory aortitis may also be present in other inflammatory diseases such as rheumatoid arthritis, (peri-)aortitis (Ormond), polychondritis, spondyloarthritis, sarcoidosis, Cogan syndrome, and Behçet disease.
This section focuses on the most common inflammatory disorders that affect the large and medium-sized arteries. Mural inflammatory changes in large and medium-sized arteries can be directly visualized by high-resolution imaging modalities. Vasculitic changes in small vessels can be visualized only indirectly. Inflammatory reactions of affected tissue, rather than the small vessel wall itself, can be demonstrated.
Imaging tools have been demonstrated to assess the extent and activity of vasculitic changes in a very sensitive way. The extent and distribution of vasculitic changes may help to narrow the potential differential diagnosis. However, inflammatory tissue reactions that can be demonstrated with noninvasive imaging may be similar in various vasculitides. For making a specific diagnosis, additional clinical or laboratory findings should be taken into account. For example, with noninvasive imaging, aortitis with subclavian stenoses caused by GCA may resemble aortitis with subclavian stenosis caused by TA. The patient’s age is frequently very useful to distinguish between the two.
Large Vessel Vasculitides
Giant Cell Arteritis
GCA, also known as temporal arteritis, is the most common large vessel vasculitis. It has an incidence of up to 20 per 100,000 inhabitants. The prevalence is higher in northern urban populations as compared with southern rural populations. Female patients are more often affected than male patients. The typical age of initial presentation ranges from 60 to 80 years. Clinical presentations vary, and clinical signs may be nonspecific. Potential symptoms include scalp tenderness, new headaches, jaw claudication, and visual symptoms such as diplopia, amaurosis fugax, or even blindness. The following American College of Rheumatology criteria can be used for classification: age at onset of disease greater than 50 years, new onset or new type of localized pain of the head, temporal artery abnormality (tenderness, decreased pulsation), elevated erythrocyte sedimentation rate (>50 mm according to the Westergren method), and abnormal findings on biopsy of the temporal artery. Temporal artery biopsy demonstrating granulomatous mural inflammation with the presence of multinucleated giant cells or a disrupted internal elastic membrane is considered the diagnostic gold standard. Once GCA is suspected, immediate steroid treatment should be initiated. Typically, patients demonstrate rapid improvement of symptoms within a few days. However, long-term steroid treatment is mandatory to prevent relapsing disease. Alternative medications such as methotrexate or infliximab have been discussed, albeit controversially, to help reduce the amount of corticosteroid administered.
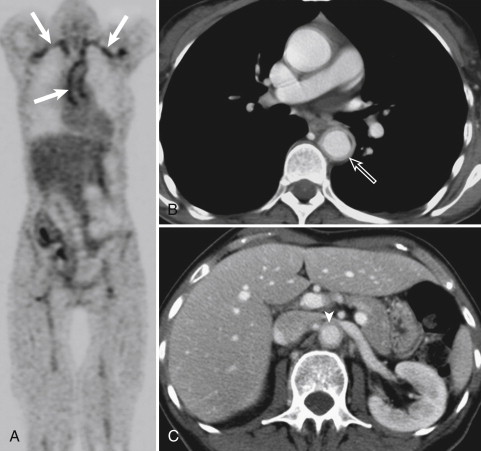
With more frequent use of noninvasive imaging modalities, the incidence of large vessel involvement has been found to be higher than previously believed. A study of 69 patients with GCA revealed presence of aortitis, for example, in 65% patients, and involvement of the subclavian arteries in 37%, followed by axillary, renal, carotid, and other large artery involvement. Noninvasive whole body vasculitis activity mapping would be desirable for assessment of vasculitis activity in therapeutic decision making.
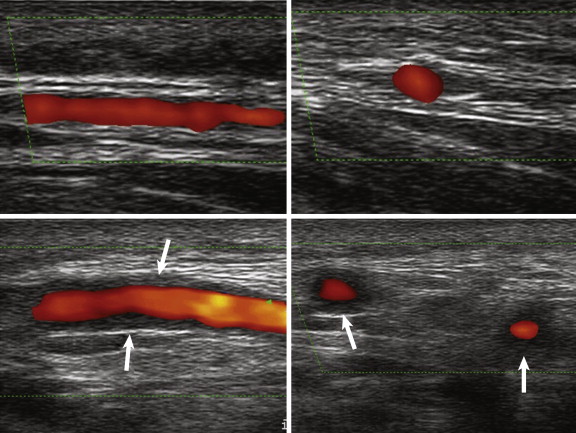
CCDS can be considered the imaging modality of first choice for detection of mural inflammatory changes in GCA. In the hands of a well-trained observer, CCDS can be a powerful tool for identifying the typical halo, flow alterations, or stenoses of the superficial temporal artery ( Fig. 25-1 ). With its high spatial resolution of approximately 100 mcm, clear depiction of the superficial cranial arteries and their mural anatomy is possible. A meta-analysis including more than 2000 patients revealed a weighted sensitivity and specificity of the halo sign of 69% and 82%, respectively, and a weighted sensitivity of stenosis or occlusion of 68% (compared with biopsy findings). CCDS is readily available, harmless, cost effective, and repeatable. However, it is observer dependent and can be rather time consuming, especially when the cranial involvement pattern and the supraaortic arteries are assessed at the same time. The thoracic aorta, which also can be involved, may not be clearly visible on CCDS because air in the lung may overlie the scan window.
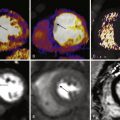
FDG PET uses glucose metabolism for visualizing inflammatory tissue. The radioactive tracer FDG is metabolized in tumor cells and active inflammatory cells. Increased metabolic activity can be detected in areas of vascular inflammation ( Fig. 25-2 ). Because of the small (picomolar) amount of tracer uptake necessary for visualization, FDG PET scanning is considered the most sensitive noninvasive imaging modality for detection of large vessel vasculitides. However, the increased metabolism is nonspecific, and FDG PET has low spatial resolution of approximately 4 mm. In addition, the underlying physiologic cerebral uptake of FDG precludes the ability of FDG PET to visualize the mural inflammatory changes of the superficial temporal arteries in GCA. Further, FDG PET is not everywhere available, it is costly, and it involves the use of ionizing radiation.
With its various tissue contrasts and the use of gadolinium-based contrast agents in imaging planes of any desired obliquity, MRI is a versatile tool for assessing inflammatory changes in many areas of the body. High-resolution MRI can noninvasively visualize mural inflammatory changes of arteries as small as the superficial temporal arteries. Typical MRI signs of vasculitic involvement include mural contrast enhancement and mural thickening ( Figs. 25-3 and 25-4 ). To depict the occasionally subtle changes associated with GCA most clearly, a specific high-resolution protocol for visualization of the superficial temporal artery wall is necessary. This protocol includes a T1-weighted, contrast-enhanced multislice spin echo sequence with the following parameters: repetition time, 500 msec; time to echo, 22 msec; spectral fat saturation; field of view, 240 × 240 mm 2 ; acquisition matrix, 1024 × 768; acquired in-plane resolution, 195 × 260 mcm; slice thickness, 3 mm; and acquisition time, 6:42 minutes at 1.5T and 4:52 minutes at 3T. Initial single center trials revealed a sensitivity and specificity of 80% and 97% and a positive and negative predictive value of 96% and 84%, respectively. Sensitivity can be increased to 86% when imaging in the first few days of steroid treatment.
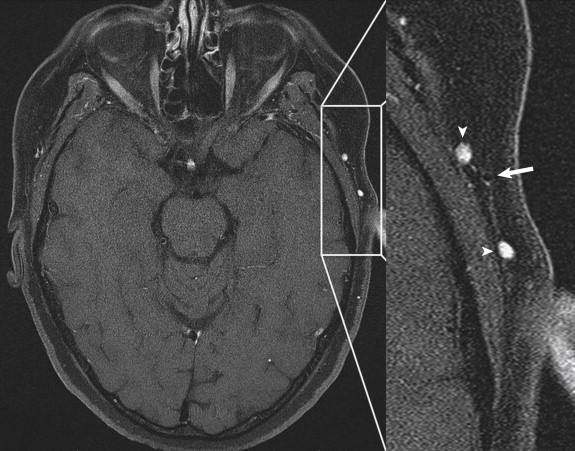
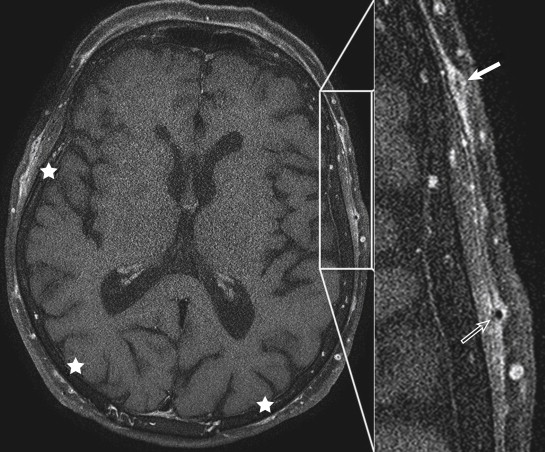
MRI follow-up studies of patients with biopsy proven GCA revealed that MRI signs were pronounced in active disease and decreased by steroid medication. This finding underlines the need for immediate scanning before steroid treatment effects diminish MRI-visible mural inflammatory changes. Relapsing disease has been visualized in individual cases in which repeated MRI scans have been performed.
High-resolution imaging of the superficial temporal arteries can be combined with MRA of the thoracic aorta and the supraaortic branches for assessment of large artery involvement within a single MRI or MRA study. This combined approach offers incremental value for the patient and the practitioner to understand the extent and activity of this systemic vasculitis.
An intraindividual head-to-head comparison of MRI and CCDS revealed that both imaging techniques had comparably high sensitivities and specificities in the detection of mural inflammatory changes in GCA. MRI demonstrated higher values for detection of disease, but differences did not reach the level of statistical significance. The intracranial and intradural arteries of the circle of Willis were not affected in a retrospective analysis of 50 patients with biopsy proven GCA and MRI signs of mural inflammation of the extracranial superficial temporal arteries. Increased contrast material enhancement of the intracranial and extradural medial meningeal artery was found in 32% of cases.
MRI images can be acquired by using a standard scanning protocol and are therefore observer independent. However, MRI is expensive and is not readily available everywhere. Published studies mainly report single center experiences. Higher levels of evidence from larger patient trials are warranted. Results from a multicenter trial based in Germany are expected soon.
Takayasu Arteritis
TA, also known as pulseless disease, is a chronic granulomatous form of vasculitis of unknown origin that affects the aorta and its major branches including the coronary and pulmonary arteries. Female patients less than 40 years old are predominantly affected. Inflammatory mononucleated infiltrates lead to mural thickening with multifocal stenosis and occlusion. Less than 10% of patients will also have dilatation and aneurysm formation of the affected arteries. Fatigue, mildly elevated body temperature, loss of weight, night sweats, myalgias, and arthralgias are typical presenting symptoms. Ischemic signs develop with progressive disease, predominantly claudication of the upper extremities and reduced cerebral perfusion, depending on the site of vasculitic manifestation. Aortic involvement is most common, followed by (in descending order) the subclavian, carotid, pulmonary, and abdominal arteries. The American College of Rheumatology criteria for classification of TA include age at onset of disease less than 40 years, ischemia of at least one extremity, reduced pulse of the radial or ulnar artery or both, systolic pressure gradient greater than 10 mm Hg between both arms, bruits over one or both subclavian arteries or abdominal aorta, and angiographic evidence of inflammatory changes of the aorta, its branches, or large extremity arteries.
Imaging in TA should ideally allow early diagnosis, including detailed assessment of the extent and severity of mural vasculitic changes and differentiation from atherosclerotic disease. Follow-up studies are used to monitor effectiveness of therapy and to identify relapsing disease. Ideally, therapy should be tailored according to disease activity, with the aims of reducing the amount of antiinflammatory medication necessary and therefore minimizing the risk of drug-induced side effects.
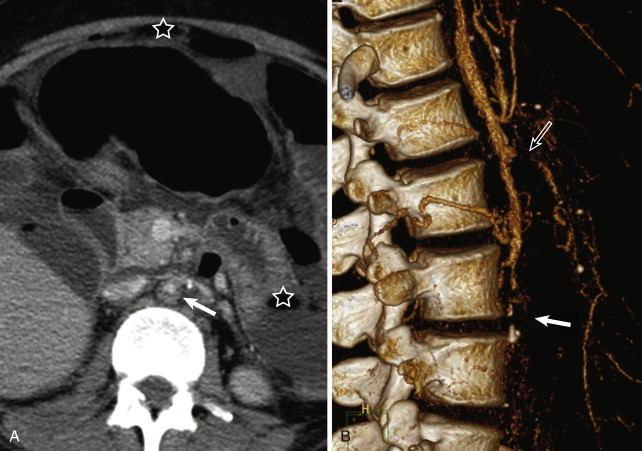
Similar advantages and limitations of the noninvasive imaging techniques CCDS, MRI or MRA, CT or CT angiography (CTA), and PET CT apply for imaging in TA and GCA. CCDS is readily available for assessment of mural thickening, luminal narrowing, and flow alterations in the carotid, subclavian, and axillary arteries. The thoracic aorta cannot be visualized fully because of the overlying lung parenchyma. DSA displays the arterial lumen in very high resolution. However, mural inflammatory changes cannot be visualized directly. CTA and MRA do not have these limitations of CCDS and DSA ( Figs. 25-5 and 25-6 ). Both CTA and MRA deliver angiographic images of the vessel lumen and can be combined with high-resolution imaging of the vessel wall in any desired orientation. Typical inflammatory changes include mural thickening and contrast enhancement. MRI, in particular, is useful for assessing the degree of vessel wall inflammation because of its various tissue contrasts ( Fig. 25-7 ). High T2 signal intensity is considered consistent with mural inflammatory edema ( Fig. 25-8 ). These mural changes may be present before the development of any vessel narrowing or dilatation. The role and diagnostic utility of increased mural T2 signal are controversial; different studies have reported varying levels of correlation between mural edema and inflammatory activity.