Interventional Endosonography
Diagnostic and therapeutic endoscopic ultrasound (EUS) has established itself as the interdisciplinary “royal discipline” of interventional endoscopy.1 The combination of ultrasound and endoscopy permits the use of high-frequency ultrasound scanning and supplemental ultrasound techniques in anatomical regions that are poorly accessible or inaccessible to transabdominal or transthoracic ultrasound. A familiarity with conventional ultrasonography combined with the extraordinary capabilities of side-viewing endoscopy are essential prerequisites for successful therapeutic endosonography.
The capabilities of EUS-guided biopsy techniques have increased the importance of EUS as a diagnostic tool. The advantage of these EUS-guided techniques is that they can accurately direct a needle under vision into a previously inaccessible target lesion located deep in the mediastinum, retroperitoneum, or pelvis.
Endosonography can play an important role in interdisciplinary planning and in the treatment of pancreatic pseudocysts, (peri)pancreatic abscesses and necrosis, pancreatic duct obstructions, biliary strictures, pancreatic duct stones, and many other minimally invasive applications while providing a remarkably low complication rate.2,3
22.1 Cost–Benefit Analysis
Today endosonography is and in the future will remain on a competitive footing with other interventional guidance modalities such as CT and MRI (based on cost–benefit analysis of personnel costs, training costs [personal experience], and equipment and operating costs [endoscope, ultrasound scanner]). Thus, because EUS requires a high degree of practical experience and the equipment entails additional investment costs (although modest relative to those for CT and MRI), it can be particularly effective in interdisciplinary settings, despite the fact that multidisciplinary frameworks are not available at every institution.
22.2 Historical Introduction
The development of endosonography began in the early 1980s, coinciding with the advent of mechanical radial ultrasound scanners and electronic linear scanners.4–6 Olympus offered the first commercially available radial echoendoscope in 1983, equipped with a motor-driven rotating sector scanner. At the same time, companies such as Siemens, Acmi, and Toshiba/Machida developed rotating sector scanners in addition to longitudinal echoendoscopes equipped with linear array transducers.7,8 In 1990, T. Rösch and colleagues published the first report on the successful use of miniaturized radial transducers passed through the working channel of an endoscope (“miniprobe”).9
Despite the appealing image quality, endosonography initially met with limited acceptance. It became widely practiced only after the introduction of EUS-guided interventions, which in a first step consisted mainly of fine needle biopsies. These procedures became possible when Picker, working with Pentax in the early 1990s, developed the first longitudinal echoendoscope with a miniaturized curved-array transducer and a working channel.10 Initial results of EUS-guided fine needle aspiration biopsies were published in 1993.11,12 Other milestones in the development of endoscopic ultrasound were the introduction of electronic radial scanners by Olympus/Aloka and Hitachi/Pentax in the early 2000s and the introduction of real-time endosonographic elastography and contrast-enhanced endosonography.13–16
The era of therapeutic endosonography was launched by Maurits Wiersema in 1996 with the first reports on endosonographic celiac plexus neurolysis and EUS-guided drainage of pseudocysts.17,18 Reports published in 2002 and 2003 described initial experience with EUS-guided biliary drainage19 and pancreatic duct drainage.20
Meanwhile, EUS-guided fine needle aspiration biopsy (EUS-FNA) has had a crucial impact on therapeutic decision-making by providing a definitive diagnosis for lesions demonstrated by endoscopic ultrasound. In Germany, an average of 15% of endosonographic procedures include fine needle biopsy, and the biopsy rates at some centers are considerably higher (up to 30%). EUS-guided therapeutic interventions enrich the spectrum of interventional endoscopy, although they are mostly limited to centers that possess the necessary expertise. Approximately 3% of endosonographies performed in Germany are therapeutic procedures. The most common procedure is the drainage of pancreatic fluid collections and necrosis (60%), followed by biliary and pancreatic duct drainage (23%), celiac plexus neurolysis or blockade (8%), and the drainage of nonpancreatic fluid collections (9%) (www.eus-degum.de).
22.3 Materials and Equipment
22.3.1 Requirements of the Endoscopy Unit
The EUS procedure room should meet the general requirements of an interventionally equipped endoscopy unit and must also have the necessary sonographic and endosonographic equipment. The following are required:
Fluoroscopy unit (fixed or portable C arm)
Video processor, cold light source
Monitors (endoscopy, fluoroscopy)
Imaging documentation systems (PACS, etc.)
Electrosurgical unit
Surveillance monitor (including continuous measurement of oxygen saturation, pulse rate, ECG leads, and blood pressure monitoring)
Oxygen port, continuous oxygen delivery
Additional suction equipment
Other necessary gear includes lead aprons, thyroid shields, and radiation dosimeters for operators and assistants. Every endoscopy unit should have an emergency kit with essential drugs as well as equipment for intubation and bag ventilation.
Optional accessories include the following:
Video duodenoscope, therapeutic forward-viewing upper GI endoscope (“gastroscope”)
Assorted guidewires (0.035-inch, 4 m)
Radiographic contrast medium (in sterile bottle or dish; e.g., Ultravist 370, Bayer HealthCare Pharmaceuticals [L-lysine amidotrizoate])
Fixed-diameter dilators (5F–9F)
Balloon dilators (6–20 mm)
Self-expanding metal stents (metal biliary stents)
0.9% NaCl (in sterile bottle or dish)
Sterile 10-mL syringes
Sterile gauze pads
Polyp retriever
Dormia basket
Polyp retrieval net, polyp retrieval basket (rotatable)
Miscellaneous polypectomy snares (rotatable)
Documentation Written documentation should cover the indication for the procedure, the scope of informed consent, premedication, the endoscopes and materials used, the course of the procedure, treatment results, aftercare recommendations, as well as endoscopic (and possibly radiologic) image documentation. Radiologic documentation includes the fluoroscopy time and radiation dose product. Nursing documentation includes patient identification data, course of the procedure, procedure time, monitoring parameters, and nursing care recommendations after the intervention.
22.3.2 Which Endosonography Systems Have Become Established?
Concurrently with the development of radial EUS techniques (not suitable for interventional use), initial experience was published on the use of electronic linear-array scanners, which initially could not be used for interventions (ACMI, Machida, Siemens, Toshiba). The first longitudinal echoendoscope (the FG 32-UA) was marketed in 1991, making it possible to direct a needle to a targeted site under endosonographic guidance (Picker/Hitachi-Pentax).10,11 Since then, longitudinal endosonography has become an established tool, owing especially to its usefulness for therapeutic interventions. The working channel should have an inner diameter >3 mm, preferably 3.7 to 3.8 mm (Pentax-Hitachi, Olympus, Fujinon).
A detailed analysis of available systems and their technical characteristics (electronic or mechanical, working channel, instrument length, tip, field of view, transducer frequency, Doppler capabilities, elastography) was recently published by Janssen21 and by Sudholt and Vilman.22 Details may be found in descriptions of individual interventional techniques.
Our own experience Today a top-level endoscopy facility should combine optimal sonographic techniques with endoscopes that have high maneuverability and interventional stability. Desirable sonographic capabilities include color Doppler scanning, contrast-enhanced imaging, and elastography.
Typical EUS instruments are illustrated in ▶ Fig. 22.1, ▶ Fig. 22.2, ▶ Fig. 22.3, and ▶ Fig. 22.4.
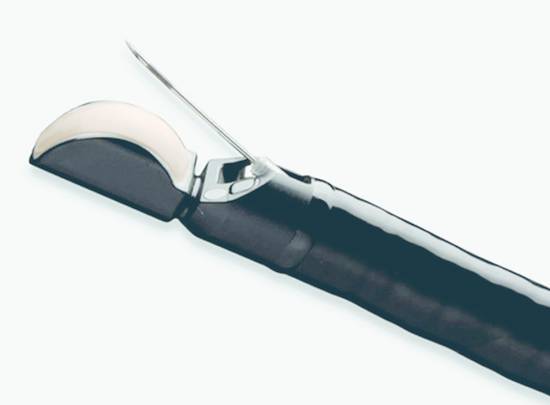
Fig. 22.1 Longitudinal echoendoscope EG 3870 UTK (Hitachi/Pentax) with fine needle deployed through a 3.8-mm working channel.

Fig. 22.2 Longitudinal echoendoscope EG 3830 UT (Hitachi/Pentax) has an approximately 45-mm-long rigid introducer section (diameter 12.8 mm) with a micro-convex probe (approximate length 20 mm, 120° scan angle, 5–10 MHz), side-viewing endoscopic lens (120°), and a 3.8-mm working channel with an Albarran lever.
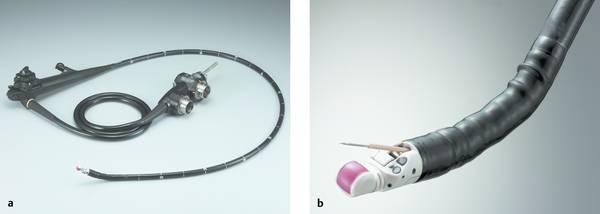
Fig. 22.3 Olympus GF-UCT180 therapeutic ultrasound gastroscope with CHE capability and 180° longitudinal scan. (Source: Reproduced with kind permission of Olympus Deutschland GmbH, Hamburg, Germany.)
a General view.
b Distal end.
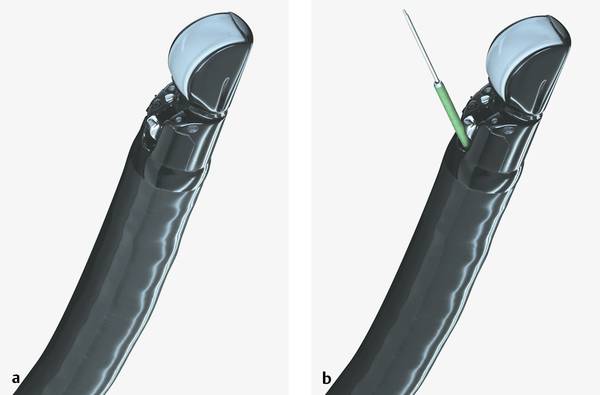
Fig. 22.4 Fujinon EG 530 UT therapeutic ultrasound gastroscope
a Without needle system.
b With needle system.
22.3.3 Which Biopsy Needles and Techniques Have Become Established?
Biopsy devices were developed in the early 1990s by Peter Vilman and Soeren Hancke in cooperation with the German endoscopic accessories manufacturer Medi-Globe Ltd. (formerly GIP Medizintechnik).
Most present-day needle systems are based on the designs introduced at that time. The first needle systems were homemade devices used as prototypes, and their successors are now available commercially from companies such as Cook Medical (wide selection of 25- to 19-gauge EchoTip aspiration needles and histology needles [Quick-Core 19G, EUSN-19-QC; ProCore 25G, 22G and 19G, Echo-HD-25/22/19C]); Boston Scientific (Expect 25G, 22G, 19G; Expect 19 Flex); Medi-Globe (ProControl aspiration needles 25G to 19G); Olympus (EZ shot 2 aspiration needles 25G to 19G); Beacon Endoscopic/Covidien (bnx system 25G, 22G, 19G). The first successful needle design widely used in studies was a metal needle system with a metal stylet (GIP, 170 cm length, 0.8 mm diameter = 22 gauge), which mounts on the instrument channel of the endoscope by a Luer lock connector. Current aspiration needle designs are characterized by a plastic coil, metal stylet, and Luer lock fitting. Trucut core needle systems are offered by Cook Medical (Quick-Core) but have not become widely implemented as first- and second-generation systems.23
In a recent innovation, developed in the United States, a 19-gauge “access needle” uses the stylet as a needle rather than a needle-protector as in conventional assemblies (EUS-19 Access, Cook Medical). When thin-lumen biopsy needles are used in echoendoscopes with a large working channel (3.7 mm), it is sometimes difficult to center the needle tip in the channel. This led Medi-Globe to introduce 25-gauge and 22-gauge needles covered by a distal sheath to help stabilize the needle tip. Beacon Endoscopic has developed the bnx needle exchange system facilitating the passage of multiple needles of various diameters (25, 22, and 19 gauge) through a single delivery system, which is kept in place during the fine needle intervention (distribution: Covidien). So far the bnx system is the only one with a needle stick prevention device.
Needles with 19-gauge diameter can be used in all longitudinal echoendoscopes even without a therapeutic working channel, although the use of a diagnostic endoscope limits interventional capabilities (e.g., stent placement is not possible without changing the scope). 19G Nitinol-needles (Boston Scientific; Beacon Endoscopic/Covidien) have higher flexibility, allowing EUS-guided biopsy and interventional procedures also in bent scope positions. Various biopsy techniques have been described, although large comparative studies have not yet been published.
On the basis of the current literature the following observation can be made:
Biopsy and cytology needles should have the smallest possible effective diameter.
Any tissue particles that are retrieved should be immersed in formalin for histologic processing.
The thin needles used to date cannot consistently retrieve histologic specimens, despite the very promising results published by some authors.
The cell block technique, based on the cytocentrifugation of aspirated material, can duplicate the advantages of histologic tissue sampling.
Cell block cytology yields tissuelike cell aggregates that can be embedded in paraffin for both histologic and immunocytologic study.24
Conventional rigid needle systems for histologic tissue sampling (19-gauge) do not (yet) have the versatile guidance needed for critical biopsy applications, especially in the pancreatic head, and are in need of further refinement.
Our own experience We have found the 22-gauge needle to be excellent for aspiration cytology. It is uncertain whether 25-gauge needles, with their presumed lower complication rate and simplified use (biopsy of hard tissues), would be advantageous for this application. For interventional endosonography we use 19-gauge needles (Cook Medical, Olympus, Boston Scientific, Beacon Endoscopic/Covidien), which can accommodate a 0.035-inch guidewire. It should be noted that the 19-gauge needles supplied by Medi-Globe, with their slightly smaller inner lumen, cannot accommodate a 0.035-inch wire, only wires with a maximum diameter of 0.030 inch.
Typical EUS aspiration needles are illustrated in ▶ Fig. 22.5 and ▶ Fig. 22.6.
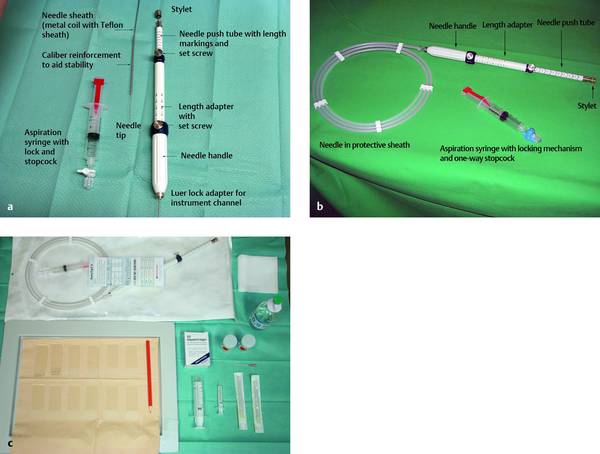
Fig. 22.5 The 22-gauge SonoTip II EUS aspiration needle (Medi-Globe). The system components are labeled (a, b) and a table setup is shown (c).

Fig. 22.6 The 19-gauge EchoTip Ultra EUS aspiration needle (Cook Medical), the standard needle for EUS-guided interventions.
22.3.4 Guidewires
Today a great variety of guidewires are available commercially. Their suitability depends on the specific indication. Radiopaque wires in lengths of 260 to 450 cm are usually flexible at the tip (made of Teflon or PVC), have a nitinol core for stability, and may have high torque stability. A hydrophilic coating facilitates wire insertion, and various color markers or centimeter scales allow for wire placement without continuous fluoroscopic guidance. The two preferred guidewires in current use are the Jagwire (Boston Scientific) and the Metro (Cook Medical) (▶ Fig. 22.7). The Terumo wire, designed for angiologic use, should be well wetted before insertion and is useful for wayfinding, especially when dealing with Klatskin tumors (HS+, FS−). The hybrid wire has a tip that is similar to the Terumo wire (continuous flush) but not quite as soft. The hybrid wire is useful after the precut and for delivering metal stents into the duodenum (the wire is 40 cm longer).
Fig. 22.7 Guidewires. The Hydra Jagwire (Boston Scientific) (a) and Metro Wire (Cook Medical) (b) are shown.
Our preferred access wires are the Jagwire and the Super Stiff wire. Both have a flexible tip that reduces the risk of perforation after insertion into the cyst lumen. In the atraumatic access technique (dilation), we have found that the Jagwire is often too unstable to provide effective guidance for a dilator. The Super Stiff wire has proven useful in this situation; it keeps the dilator in line with the dilation axis, creating an optimum vector for force transfer. One disadvantage of the conventional Super Stiff wire is the steel core of the fixed shaft; as a result the wire is not kink-resistant and must be used with caution. An improvement is the super-stiff cyst access wire developed by the firm MTW Endoskopie. This wire resists kinking, but its short length prevents the use of certain accessory devices (e.g., colon dilation balloon). Also, the wire is not insulated so it is not suitable for electrosurgical access with a cystotome or diathermy ring, for example. Medi-Globe offers a similar stiff steel wire (length 400 cm) with a flexible tip, but this wire is uncoated too, and therefore is not suitable for diathermy use.
Both the Jagwire and Metro wire are suitable for access with electrosurgical cutting instruments such as a needle-knife papillotome or the newly developed diathermy ring with circular cutting edge. The tissue can be cut with very little force application, and the wire coating is compatible with a diathermy current.
One disadvantage of coated wires is that difficult placement maneuvers (e.g., EUS-guided biliary drainage) or attempts to adjust the wire position may shear off the Terumo tip or the Teflon sheath at the sharp needle tip, which would jeopardize the success of the intervention and may lead to complications (▶ Fig. 22.8).
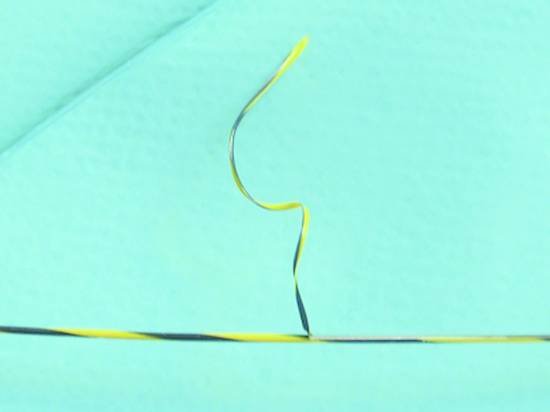
Fig. 22.8 Endoglide coating has been sheared off the guidewire by the sharp tip of a 19-gauge needle during the transgastric EUS-guided placement of a biliary drain.
A special 19-gauge intervention needle assembly (EchoTip Access, Cook Medical) with a blunt hollow needle and pointed stylet has recently been developed to minimize this risk (▶ Fig. 22.9).
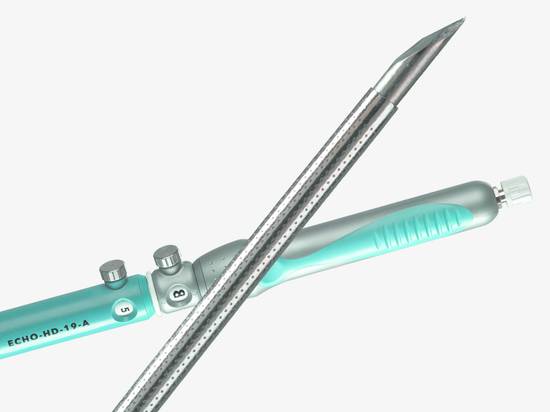
Fig. 22.9 Access needle (Cook Medical). This 19-gauge access needle uses the stylet as a needle (rather than protecting the needle with the stylet as in traditional designs).
As an alternative to a 19-gauge needle, diathermy devices (diathermy rings, cystotomes, needle knives) can be used to establish primary access to pancreatic and nonpancreatic fluid collections. Various manufacturers offer these devices, as well as fixed-diameter and balloon dilators of various diameters for enlarging a wire-secured access tract. The specific device will depend on the targeted lesion and the characteristics of the access route.
22.3.5 Fixed-Diameter Dilators
Tissue dilators ranging from 5F to 9F (▶ Fig. 22.10) can provide atraumatic access following needle insertion and wire placement. As a rule, the transgastric access route established by the guidewire is initially enlarged with a 6F biliary dilator. Because the 5F and 6F dilators have identical tips, the greater stability of the 6F dilator is better for tract enlargement. Successful dilation to 6F is followed by a second dilation with a 7F dilator, which will establish sufficient access for inserting a balloon dilator. We have also had good experience with graduated dilators such as the Cotton dilator (5–7–8.5F, 5–7–10F; Cook Medical).
Fig. 22.10 Dilator (MTW Endoskopie).
22.3.6 Balloon Dilators
The most suitable access balloon for cyst drainage is a multidiameter balloon such as the CRE balloon dilator (Boston Scientific). This type of device differs from single-diameter biliary balloon dilators (▶ Fig. 22.11, ▶ Fig. 22.12).
Fig. 22.11 CRE balloon dilators (Boston Scientific).

Fig. 22.12 Titan balloon dilators (Cook Medical).
Cook Medical now offers a similar device (Quantum TTC). The balloons are introduced in a very low-profile configuration on the delivery system permitting easy access after initial dilation to 7F or initial cutting with a needle-knife papillotome or diathermy ring. Because no waisting occurs at the center of the balloon, care should be taken on initial access that the balloon is centered in the stomach wall and does not slip into the cyst lumen or displace outward. If this occurs, we recommend deflating and repositioning the device, which is often made easier by creating an artificial waist in the balloon. The Titan balloon dilators (Cook Medical) inflate uniformly from the proximal and distal ends through a multiport system, making it less likely that the balloon will slip if not optimally centered.
The necessary balloon size depends on the proposed intervention. For example, a 6-mm biliary balloon dilator is usually adequate for cholangiodrainage. A 6–7–8-mm multidiameter balloon dilator can provide good initial access for a simple cyst drainage or planned cystoscopy. On the other hand, an emergency situation requiring immediate intervention and endoscopic débridement may require dilation to at least 12 mm, if not to 15 or 20 mm. Redilations or extensive interventions may require up to 35 mm for sufficient access. Since there are no balloons designed specifically for that purpose, an achalasia balloon dilator must be used.
The CRE wire-guided balloon dilation catheter can produce three distinct pressure-controlled diameters. Balloon sizes are printed on each package and labeled hub. The CRE balloon dilation catheter is designed to fit through the working channel of an endoscope and can accommodate a 0.035-inch guidewire. This catheter is supplied with a preloaded 0.035-inch flexible-tip guidewire in its guidewire lumen. The guidewire is 25 cm longer than the balloon catheter, with excess length protruding from the catheter hub. For safety, balloons should not be reinflated or tested before use.
22.3.7 Plastic Stents (Pigtail)
Two main plastic stent designs are used: double-pigtail stents with a loop at both ends and straight stents with or without side holes (▶ Fig. 22.13). Both types have advantages and disadvantages for cyst drainage as described below. Single-pigtail stents do not have a role in endosonographic interventions. Straight stents have better drainage properties because the absence of a curve makes the lumen less susceptible to clogging. Drains with side holes (Amsterdam stents) have the best drainage properties but poorer site stability because they generally require an inner and outer barb for retention. To solve this problem, “Tannenbaum” (Christmas tree) stents were developed that have four barbs at each end for more stable retention.
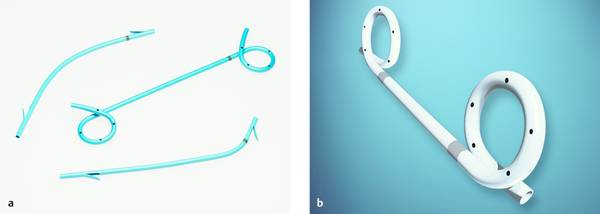
Fig. 22.13 Innovative plastic stents. The photograph illustrates a double-pigtail plastic stent along with conventional straight and angled designs (Advanix, Boston Scientific) (a). The double-pigtail stent (Solos, Cook Medical) in (b) has two radiopaque markers; it is available in 10F with the Solus introducer set for one-step placement. The set includes the radiopaque 10F stent, 10F/165-cm delivery catheter, and 5F/210-cm guide catheter. A 0.035-inch guidewire is recommended.
We generally use 8F double-pigtail stents, as we have found that the actual drainage efficiency of the stent is of minor importance for cyst drainage or even necrosectomy. The drainage effect depends on the size of the access tract that has been created, and so the function of the stent is basically reduced to maintaining tract patency. Double-pigtail stents have the best retention properties because they are atraumatic on the cyst side and the pigtail provides a good anchoring effect in the cyst and in the stomach.
It is best to use a stent with center and edge markers because following deflation of the balloon, the draining cyst fluid will obscure vision of the puncture site and increase the risk of advancing the stent too far into the fluid collection.
22.3.8 Metal Stents
Traditional biliary stents (Amsterdam stents) can be used for EUS-guided biliary and pancreatic duct drainage. The stent sizes most often used for these applications are 7F or 10F. It is important to have an adequate stent length in the liver or stomach, as movements of the stomach are apt to cause stent migration. The use of covered metal stents has recently been advocated (▶ Fig. 22.14, ▶ Fig. 22.15). These devices have the advantage of a large lumen and lower occlusion rates.

Fig. 22.14 WallFlex metal stent.
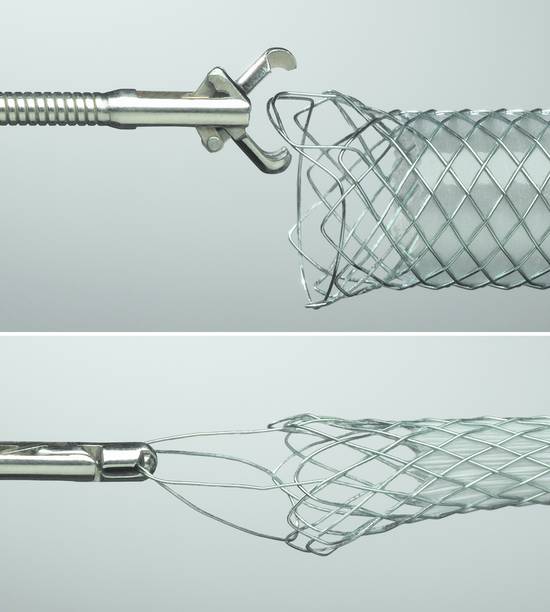
Fig. 22.15 WallFlex metal stent with forceps retrieval option.
Biliary metal stents are available in many designs, either with or without covering. Most stents are made of nitinol because of its outstanding properties but differ in their weave and wire gauge; these differences cause differences in X-ray visibility. Some stents are cut from nitinol in a way that prevents shortening during deployment. Metal stents with a thin wire weave usually carry additional radiopaque markers to ensure adequate visibility. For transgastric, transduodenal, or transjejunal EUS-guided biliary drainage, only covered stents should be used, however, in order to avoid bile leakage. Metal stents should also have some reserve length in the liver or stomach to prevent migration.
Some authors are currently testing the use of large-bore metal stents to maintain access for pseudocyst drainage or necrosectomy. This can be done with short esophageal metal stents (Ultraflex 18 mm, length 7 cm [Boston Scientific], Aixstent Esophagus 102–20–060, length 60 mm [Leufen Medical]), or specially developed pseudocyst access stents (e.g. Aixstent Pseudocyst, diameter 10 and 14 mm, nitinol, length 20 mm, fully covered [Leufen Medical]). Initial reports confirm better access for necrosectomy. The stent is only temporary, however, and must be retrievable at the end of the procedure. K. Binmoeller and his group have developed two innovative devices for endosonographic drainage: a needle- and balloon-assisted access system that employs two guidewires (Navix); and a short, covered apposition stent (Axios), also available as a combined system from Xlumena, Inc.
The unique Platinol wire construction of the WallFlex RX biliary stent system (▶ Fig. 22.14, ▶ Fig. 22.15) is setting new standards with its characteristics:
Flexibility to aid placement in tortuous anatomies and maintain luminal patency
Full-length radiopacity to improve visibility during stent placement
Radial force to maximize the stent diameter
Yellow marker on the end of the stent
Reconstrainability of the stent up to 80% of deployment to aid in repositioning
Multiple markers to increase placement accuracy
Ability to remove fully or partially covered stents to meet acute requirements
Platinol wire construction
Other features include:
Integrated retrieval loop (fully or partially covered)
Rounded and flared stent ends to resist migration
Closed-cell design and coating options
Permalume covering
Biliary RX delivery system
Four fluoroscopy markers
Yellow transition zone on the catheter
Repositioning limit marker on the handle
22.3.9 Diathermy Devices, Cystotome
Occasionally, wire-guided access to a pseudocyst, necrotic area, or duct cannot be achieved with dilators. In this case various diathermy devices, known mostly from their use in endoscopic retrograde cholangiopancreatography (ERCP), can be used following needle-assisted wire placement (▶ Fig. 22.16, ▶ Fig. 22.17a). These devices consist of monopolar needle knives delivered over a guidewire (this is essential; otherwise access to the cyst would be lost after the cut), circular metal rings or cones mounted at the tip of a wire-guided catheter (cystotome or diathermy ring), and a device that combines a central cutting wire with a larger-diameter cutting ring. Generally these instruments are powered by a software-controlled high-frequency current (Endocut). Access to a fluid collection or obstructed duct is achieved through a combination of electrosurgical tissue destruction and a mechanical pushing force.
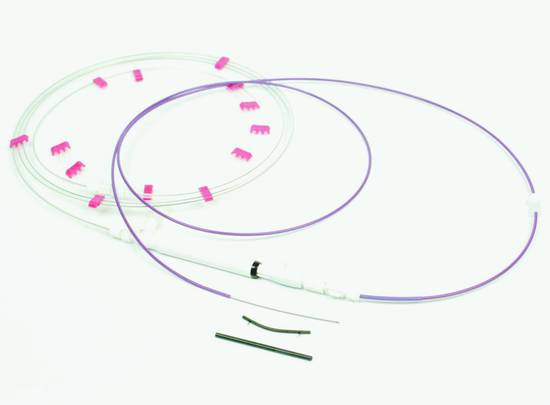
Fig. 22.16 Giovannini set (Cook Medical).
Fig. 22.17 a–c Cystotome. CST-10 cystotome (Cook Medical) for electrosurgical transgastric or transduodenal cystoenterostomy of a pancreatic pseudocyst visibly bulging into the gastrointestinal tract. The system has a minimum working channel 3.7 mm in diameter, a 10F/165-cm outer catheter, a 5F/190-cm inner catheter, and a 10F diathermy ring. The manufacturer recommends the Tracer Metro Direct guidewire (0.035-inch). The handle has two separate HF ports in contrasting colors (step one: white port; step two: black port for the 10F diathermy ring).
Wire guidance is necessary to ensure that the cutting device does not stray from the intended target.
Caution
Note that the gas generated by electrosurgical tissue destruction may cause significant artifacts, which are particularly troublesome in EUS-guided cholangiodrainage.
Suitable instruments are double- or triple-lumen needle-knife papillotomes (Huibregste HPC-3 Triple Lumen Needle Knife [Cook Medical], KD-V 441 M triple-lumen needle knife [Olympus]; Microknife XL triple-lumen needle knife [Boston Scientific]; BII GSP 34 20 020 wire-guided papillotome [Medi-Globe], diathermy rings (e.g., MTW Endoskopie), and other BII papillotomes. We prefer the Cysto-Gastro Set (Endo-Flex) without a central cutting wire, available in diameters of 6F, 8.5F and 10F, or the cutting ring cystotome (Endo-Flex) recently developed by U. Will. One disadvantage of using multilumen needle-knife or BII papillotomes is the space between the guidewire and needle, which may make it difficult to gain entry into the cyst in some circumstances.
A cystotome (CST-10, Cook Medical), ▶ Fig. 22.17) or needle-knife papillotome can also be used primarily, without prior needle insertion, when introduced under endosonographic guidance. Needle-based access is definitely safer, however, because the needle knife is not kink-resistant and may skid off the firm cyst wall after passing through the intestinal wall.
Cook Medical developed an all-in-one system in which a cutting wire is used to create initial access followed by the placement of a dilator and an 8.5F stent (“Giovannini set,” ▶ Fig. 22.16).
A very similar cyst drainage set was designed by Dr. C. Grotjahn (MTW Endoskopie). Once the diathermy needle has entered the cyst lumen, however, it must be replaced by a flexible wire before the preloaded straight 8.5F stent can be delivered. Unfortunately, the access obtainable with this system is no larger than the stent diameter, so that no further interventions can be carried out. Another disadvantage is the relatively high risk of primary or secondary migration of the relatively short, straight plastic stents.
A new development is the diathermy ring produced by MTW Endoskopie. This is a cutting knife with a circular (tapered cylindrical) cutting edge that surrounds the guidewire. The wire keeps the ring centered on the target and creates access comparable to a 6F dilator, facilitating further interventions.
Despite a lack of supportive data, the main difference between atraumatic dilation and diathermy access lies in the potential for hemorrhage. In theory, the dilator compresses the vessels in the stomach wall to minimize bleeding risk, while a cutting process may increase the risk of vascular disruption.
Cystotomes (fistulotomes) are available from Cook Medical (CST-10 10F cystotome [necessary working channel 3.7 mm], the Endoflex 2-stage fistulotome model XS 99900000340 [10F] and XS 99900000341 [8.5F]), and the diathermy ring cystotome designed by Uwe Will is available from MTW Endoskopie.
The triple-lumen needle knife (Olympus) has separate channels for a guidewire, cutting wire, and contrast instillation. Other features are a radiopaque tip and a needle knife 5 mm long and 0.2 mm in diameter.
22.3.10 Retrievers
Stent retrievers are wire-guided flexible metal coils that have a threaded conical tip (▶ Fig. 22.18). The tip is “screwed” into the distal end of a straight plastic stent by rotating the instrument shaft clockwise. This allows plastic stents to be removed through the working channel while the endoscope and guidewire remain in place. Combined with a stiff guidewire, this instrument can also be used in EUS-PD and pseudocyst drainage to mechanically enlarge a primary needle tract through fibrotic pancreatic tissue or a tough pseudocyst capsule.
The Soehendra stent retriever (SSR-x, Cook Medical) is available in diameters of 5, 7, 8.5, 10 and 11.5F. It is designed primarily for engaging and removing biliary and pancreatic duct stents with the guidewire still in place. It is particularly useful for pancreatic duct drainage performed during an EUS-guided intervention. The minimum working channel matches the corresponding French size (▶ Fig. 22.18).

Fig. 22.18 Soehendra retriever (Cook Medical).
22.3.11 Supplementary Techniques in EUS-guided Biopsy
The success of an intervention depends critically on the personal experience of the operator in ultrasonography and endoscopy (which includes institution-specific features), the puncture technique including specimen collection (number of needle passes), and specimen processing in cooperation with the cytologist (cellularity) or pathologist (formalin fixation, correct identification of tissue fragments versus blood clots). Supplies should include an adequate number of glass slides (>5) with a frosted end for labeling, formalin solution for tissue fixation, isotonic (0.9%) saline solutions, and special media.
Special tests are applied selectively, such as immunocytochemical tests and FISH in lymphoma diagnosis, PCR and culture for detection of tuberculosis, and tumor marker assays (especially CEA but also CA19–9, AFP, CA15–3) in fluid samples from, say, cystic pancreatic lesions for clinical and chemical evaluation (cell differentiation, tumor markers, microbiology, etc.). Attention should be given to various cytologic methods. Wet fixation for cytologic analysis is necessary only if Papanicolaou staining is to be done. But many cytologists now use the simpler May–Grünwald–Giemsa stain, which requires simple air-dried slides. It should be noted that immunocytology also requires air-dried slides because a wet-fixed specimen will not take additional staining. Detailed clinical information is necessary for a discriminating analysis (genetic analysis, proliferation indices). Accurate lymphoma classification requires the examination of a whole lymph node because some lymphomas can be correctly classified only by their infiltration pattern in specific lymph node regions.
22.4 Procedure
The patient is generally placed in a left lateral decubitus position that is turned slightly toward the table to reduce aspiration risk. The patient may be positioned prone for papillary lesions or, less commonly (also due to aspiration risk) supine for pancreatic drainage.
22.4.1 Sedation
The goal of sedation is to immobilize the patient so that the technically challenging intervention can be carried out. Sedation is induced by the fractionated administration of Disoprivan (Propofol in initial dose of 40–60 mg), which may be supplemented if necessary by midazolam (e.g., Dormicum [Roche Pharma] 1–2.5 mg). Pulse oximetry, ECG, and blood pressure monitoring should be supervised by a physician experienced in critical care medicine (Chapter ▶ 11). Peri-interventional oxygen supplementation is standard at our facility. Generally there is no need to add topical oropharyngeal anesthetics (e.g., Xylocaine [lidocaine] spray [AstraZeneca]) or analgesics such as pethidine hydrochloride (Dolantin, Sanofi).1,25
22.4.2 Other Medications
The administration of N-butylscopolamine (Buscopan, Boehringer Ingelheim Pharma) or atropine is necessary only in exceptional, justified cases. Antibiotic prophylaxis follows published recommendations and is mandatory in the puncture of cystic lesions. Suitable antibiotics are aminopenicillins (e.g., amoxicillin IV or oral, 3 × 1 g), cephalosporins (e.g., ceftriaxone 2 g IV, single shot), and fluoroquinolones (ciprofloxacin or levofloxacin, 400 mg IV, 500 mg or oral twice, but only on the intervention day).
22.4.3 Orientation
The patient’s neck should be slightly hyperextended during insertion of the echoendoscope (due to the relatively stiff instrument tip). The procedural protocol and orientation are not standardized. We have found it helpful to advance the scope under combined endoscopic and endosonographic guidance. Possible complications have been described in detail and should be avoided.26,27 Mural lesions may be approached under endoscopic vision, though this is not mandatory. Whenever possible, we rely more on endosonographic than endoscopic orientation. Generally it is unnecessary to distend the stomach with water, and we do not use balloon distention.
22.4.4 General Rules for Needle Insertion
EUS-guided drainage generally employs an echoendoscope with a large-bore working channel (3.7 mm or 3.8 mm, depending on the manufacturer) and an Albarran lever. When an echoendoscope with a small working channel is used, the initial puncture must be made with a 22-gauge needle. After insertion of a 0.025-inch guidewire, the initial scope is then exchanged for a therapeutic duodenoscope or gastroscope so that further therapeutic steps can be carried out. A 22-gauge needle is generally adequate for EUS-guided injection therapy. The ultrasound platform should permit color duplex scanning to ensure a high degree of procedural safety.
22.4.5 Biopsy Technique
After the targeted lesion has been identified, the ultrasound probe is brought to bear on the lesion and placed in a stable position (artifact-free endoscope coupling with no troublesome air echoes). Because the access angle is relatively flat, it is helpful to adjust the control wheel to position the lesion at the top of the image before the needle is introduced. Color duplex scan can exclude interposed blood vessels. The metal coil or Teflon sheath (together with the retracted needle) is then introduced into the biopsy channel. The needle should be fixed at this point to avoid damage to the EUS probe. The coil is introduced completely, and the handle is firmly connected to the endoscope by the Luer lock mechanism. With adjustable-length needles, the length should be adjusted so that the metal coil or Teflon sheath of the needle is just visible sonographically and/or endoscopically. If a needle with Teflon sheath is advanced too far, it may accidentally perforate the sheath (▶ Fig. 22.19). In this case a wire can no longer be passed when the needle is withdrawn. The working channel may also be damaged during needle removal.26
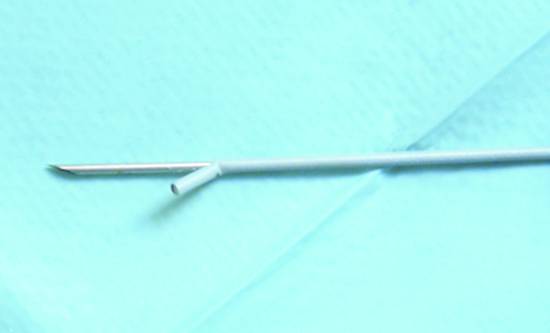
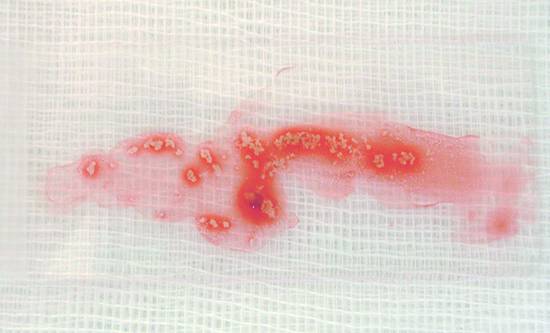
Fig. 22.19 The Teflon sheath of this 19-gauge EchoTip aspiration needle (Cook Medical) was perforated by the sharp steel needle during the aspiration biopsy of a pseudocyst.
To perform the biopsy, the blunt stylet inside the fixed needle is withdrawn by about 10 mm so that the sharp point can be advanced by 6 to 8 cm (or farther in some cases). In needles with a pointed stylet, it is unnecessary to withdraw the stylet. On reaching the target lesion, some authors recommend readvancing the stylet to reduce contamination of the aspirate by mural epithelial cells. Other authors reject this technique on hygienic grounds, since advancing any object from the needle constitutes contamination. When the needle tip has reached the target lesion, the stylet is removed and suction is applied to the needle with the 10-mL Luer lock syringe. At this point the needle is repeatedly moved back and forth in the lesion under constant EUS vision to aspirate cells and tissue. Any suction that is applied should be discontinued before the needle is removed to avoid aspirating nonlesional tissue and luminal fluid. Other authors do not use a stylet or suction at all (proving that even this technique can yield acceptable results). After the biopsy is completed, the needle is first retracted into the sleeve and then removed from the working channel together with the sleeve and handle.
To sample a different site in a presumably malignant lesion, the needle should be changed. If blood residues are sampled, the needle should be flushed between passes with sterile saline solution and then “blown out” with an air-filled syringe to avoid fluid-induced artifacts due to cellular swelling.
22.4.6 Suction
Aspiration techniques (with or without suction) vary greatly in clinical practice. Biopsy without suction is based on the experience published by Wallace et al that applying suction during the EUS-FNA of lymph nodes increases the blood contamination of smears and therefore does not improve the diagnostic yield despite the greater cellularity.28 On comparing intermittent and continuous suction as well as aspiration syringes of different volumes, Bhutani et al found that continuous suction with smaller syringe volumes (5–10 mL) provided an optimum cell yield.29 It may be that aspiration at higher suction can improve the recovery of material suitable for histologic evaluation.30,31
22.4.7 Specimen Processing
The stylet is slowly advanced within the needle to expel cells and any tissue fragments onto a tilted glass slide held beneath the needle tip. The material is then smeared, air-dried, and processed further for cytologic examination. Small tissue cores or formed clots are placed in formalin solution for histologic evaluation (▶ Fig. 22.20, ▶ Fig. 22.21, ▶ Fig. 22.22, and ▶ Fig. 22.23). Procedural details and problems relating to blood in the aspirate and specimen processing are described elsewhere26,32 (see Chapter ▶ 6).