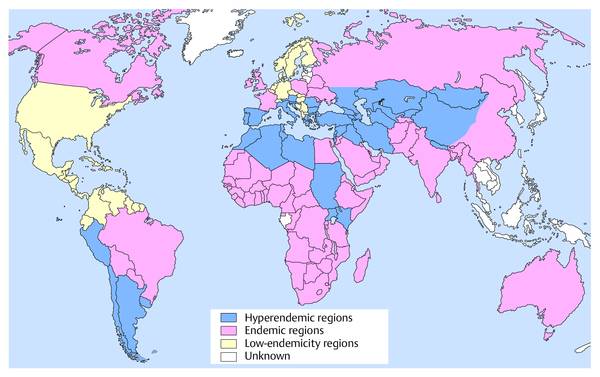
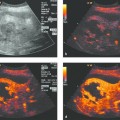
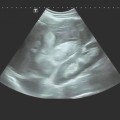
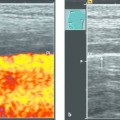
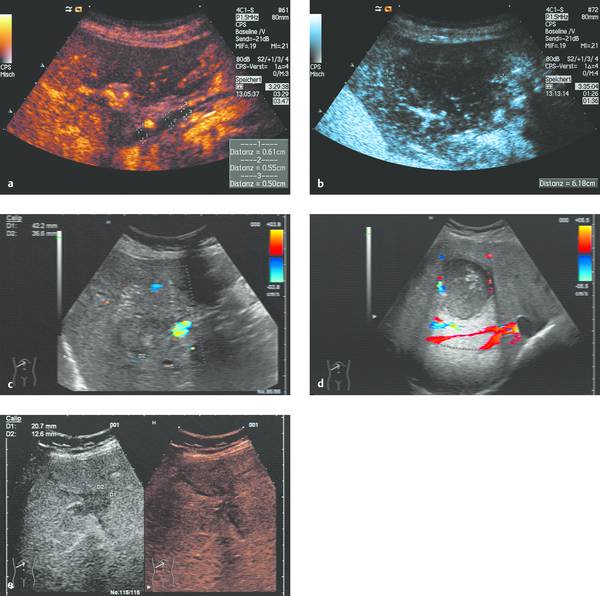
Interventional Treatment of Echinococcosis Echinococcosis (hydatid disease) is a zoonotic infection caused by flatworms of the class Cestoda. The dog tapeworm (Echinococcus granulosus or cysticus; cystic echinococcosis) and fox tapeworm (Echinococcus multilocularis or alveolaris; alveolar echinococcosis) can cause parasitisms in humans. Two other species that are pathogenic to humans (Echinococcus vogeli and Echinococcus oligarthus) are confined to Central and South America and are very rare. An improved understanding of hydatid disease and advances in imaging technology have revolutionized the diagnosis, treatment, and prognosis of cystic echinococcosis. This does not apply equally to alveolar echinococcosis, which unfortunately continues to be a diagnostic and therapeutic “chameleon.” Cystic echinococcosis—the main topic of this chapter—is generally diagnosed by an imaging study (ultrasound or possibly CT) combined with serologic techniques (ELISA and confirmation tests). A WHO working group has assembled the various classification systems to provide a summary of morphologic findings that are relevant to treatment. The WHO guidelines and recommendations for establishing a uniform approach to the diagnosis and treatment of echinococcosis have been published.1–3 Echinococcus granulosus (the dog tapeworm) is the main causative agent of acquired parasitic (cystic) liver disease in Europe. Its prevalence increases markedly from northern to southern latitudes (▶ Fig. 17.1). The incidence of symptomatic cystic echinococcosis varies considerably worldwide and, depending on the region, ranges from <1 per 100,000 population and year (estimated 0.5/100,000 in Germany) to 198 per 100,000 a percentage range/prevalence in certain regions based on living habits, as in northwest Kenya (Turkana) in Africa.4–7 Obviously there is a high percentage of unreported, asymptomatic cases in which the parasites produce no clinical manifestations. The cycle of transmission through dogs and sheep explains why endemic cystic echinococcosis is most prevalent in sheep-raising regions: Anatolia (Turkey), Eastern Europe, (southern) Mediterranean countries (North Africa, Asia Minor) and South America. By contrast, cystic echinococcosis is a rare disease in Central and Northern Europe and is diagnosed almost exclusively in guest workers and immigrants (and rarely in travelers). Unlike the dog tapeworm, whose larva grows by expansion, the larva of Echinococcus multilocularis (fox tapeworm) grows by infiltrating and destroying surrounding tissues (▶ Fig. 17.2). What may look like a cyst is actually a central necrotic zone within the parasitic tissue. As with the dog tapeworm, infection is acquired by the gastrointestinal route and the larvae are deposited chiefly in the liver. The geographical regions most commonly affected in Central Europe are Baden-Württemberg, Bavaria, Tyrolia, Kärnten, Styria, and Switzerland. The field mouse is a common intermediate host. Fig. 17.2 Echinococcus multilocularis. a, b Larva of Echinococcus multilocularis (fox tapeworm) infiltrating its surroundings. With contrast-enhanced ultrasound, the left lobe of the liver shows less enhancement while portions of the liver show no vascularity. The left hepatic artery is occluded and is visible between the markers. Similar changes are found in cholangiocellular carcinoma, which may also be associated with liver infarction in some cases. c–e Later image sequence documents response to pharmacologic therapy. c Ultrasound scan of the hepatic right lobe shows an echogenic, inhomogeneous mass at the center of the lobe displacing the surrounding vessels, consistent with untreated Echinococcus multilocularis. Indistinguishable from a tumor mass by ultrasound. d Appearance of the same finding after 2 months of albendazole therapy. Note the inflammatory peripheral hypervascularity of the lesion with complete central liquefaction due to dead parasites. e Same site after 6 months of therapy. Ultrasound shows a significantly smaller mass with a nonenhancing area in the portal venous phase after contrast administration. The lesion is no longer visible in an unenhanced scan. The clinical manifestations of alveolar echinococcosis result less from mass effects than from hepatic complications such as cholestasis or chronic cholangitis. Itching, skin rash, and night sweats are typical associated symptoms. Alveolar echinococcosis is most often confused with hepatocellular carcinoma, but forms that mimic hepatic cirrhosis have also been described. Abscess formation is not uncommon, especially after the initiation of treatment. The disease may also present with pyloric stenosis, obstructive jaundice, or portal hypertension. Because Echinococcus multilocularis is not suitable for ultrasound-guided interventional treatment due to its exophytic growth and relatively solid consistency, that entity will not be discussed further in this chapter. The only treatment options are surgical removal or albendazole therapy (▶ Fig. 17.2). Caution Echinococcus multilocularis is not suitable for ultrasound-guided percutaneous treatment. Symptoms in echinococcosis depend on the location and size of the cyst (generally must be >8 cm in the liver, considerably smaller in other organ systems [brain, eye]) and may result from complications. Large cysts may cause compression with an associated feeling of abdominal fullness, epigastric pain, or nausea. Cyst rupture may have a spontaneous, traumatic, or iatrogenic cause (due to puncture or drug-induced involution) and may or may not incite an allergic reaction or occasional anaphylaxis. Other possible complications are fistula formation between hydatid cysts and bile ducts (or bronchi), for example, and symptoms due to secondary obstruction and inflammation (cholangitis, bronchitis). Spontaneous fractures have been reported due to skeletal involvement and erosive hemorrhage due to vascular infiltration. A feared complication is dissemination of the parasite into the abdominal or thoracic cavity from a ruptured cyst. Apparently it is much more common for the cysts to undergo spontaneous involution and transformation to an inactive, calcified state. Any of the following complications may arise from cystic echinococcosis: Cyst rupture (spontaneous, traumatic or iatrogenic), with or without secondary dissemination Fistula formation Obstruction and inflammation The diagnosis of echinococcosis is based on the history (nationality, exposure risk), imaging findings, and serologic testing. Abnormal laboratory parameters reflect organ involvement and its complications and are often unhelpful in making a diagnosis. Liver function and cholestasis indicator enzymes may be abnormal in patients with hepatic involvement. Significant eosinophilia is rarely observed (<10% of cases) and, in contrast to the diagnosis of helminthiases, is not a helpful finding. ELISA test systems and the indirect hemagglutination antibody test (IHAT) are sensitive screening tests that are usually combined and can confirm a positive Western blot with high specificity. The value of imaging studies depends on the location of the cyst. Ultrasonography is the imaging modality of choice for the diagnosis of abdominal cystic echinococcosis and its follow-up, while CT is indispensable for evaluating the lung and bone. The sonomorphologic staging classification described below can be applied to most organ systems. Various classification systems have been published.8–13 In 1981 Gharbi developed a classification of hydatid morphology10 that has been modified several times over the years. A Gharbi type I cyst is a pure fluid collection that is difficult to distinguish from other types of epithelial liver cyst. It is the most common lesion (50–80%, depending on the study population), and its morphology is that of a “normal” cyst without internal septa or echoes (hydatid sand). The cyst contains fertile protoscolices. The main difficulty lies in distinguishing it from an epithelialized cyst. The hallmark of Gharbi type II cyst morphology is a split wall with separation of the membranes, which may fill the interior of the cyst producing a “water lily sign” on ultrasound. The cyst is fertile and is basically a spot diagnosis on ultrasound images. Ultrasound is definitely superior to other modalities at this stage for depicting the fine details of the cyst wall and contents. The more membranes and hydatid sand there are in the cyst, and the more difficult the scanning conditions or poorer the equipment, the greater the likelihood that this stage will be mistaken for an abscess or neoplasm. The detection of hydatid sand is aided by moving the patient to a lateral decubitus or standing position. The Gharbi type III cyst is characterized by daughter cysts with internal septa and the possible presence of degenerated solid material. The septa eventually produce a honeycomb pattern with undulating membranes. This stage is called the transitional stage because live parasitic tissue undergoes regressive changes that may occur spontaneously or in response to host defenses or drug therapy. Typical features are a “cyst within a cyst” pattern (www.EFSUMB.org14]), hydatid sand, and stippled contrast enhancement in the surroundings, which becomes more distinct from stage I to stage III. The Gharbi type IV cyst has a very heterogeneous echo pattern with mixed high- and low-amplitude echoes (regressive changes due to scarring). Characteristic ultrasound signs of an inactive lesion include a collapsing, somewhat flattened, elliptical cyst shape (correlating with low intracystic pressure) and detachment of the germinal layer from the cyst wall (water lily sign). Other signs include coarse intracystic echoes and initial calcification of the cyst wall (see type V). It may be difficult in some cases to exclude the complex late stage of an abscess. This stage is much less typical than stages II and III. A Gharbi type V cyst has a calcified wall and contents. This stage reflects mixed expansile changes (perifocal reaction) and regressive scar contraction showing highly variable echogenicity with a calcified wall and internal structures. The calcified rim may create an “eggshell” appearance on CT, which can also be demonstrated sonographically. Similar morphologic changes may develop in the lung, in bone, and rarely in the brain. An improved morphologic and functional classification takes into account not only imaging characteristics but also the biological behavior of the parasites—viable (fertile) protoscolices (Garbhi types I and II) versus inactive, nonviable (infertile) parasitic cysts that have undergone degenerative and regressive changes (Garbhi types IV and V). The transitional stage (Garbhi type III) cannot be uniquely classified in terms of its biological behavior. It is significant that one lesion may show various developmental stages. The evolution of cyst morphology takes 5 to 10 years to reach the regressive, calcified end stage. It has been found, however, that calcifications may also be observed during the initial (viable) stage. It became necessary to unite the different staging classifications into a uniform system. Vivid descriptions of pathognomonic ultrasound signs have been published in the literature but should be discarded in favor of a precise nomenclature. The double membrane has been described as the “double line sign”; mobile echogenic cyst contents as hydatid sand or a “snowstorm” pattern; separation of the endocyst as the “water lily sign”; and multiple internal septa as a rosette sign or honeycomb pattern. The WHO classification published in 20011,3 not only describes the stages of cyst evolution but also subdivides echinococcal cysts into three groups to aid in clinical viability assessment and follow-up (▶ Table 17.1). This WHO ultrasound classification of cystic echinococcosis compares the sonomorphologic classifications of Gharbi, Perdomo, and Caremani,15 correlates them with the WHO stages, and provides a clinical subdivision of echinococcal cysts into three groups—active, involuting, and inactive—that are useful for treatment planning and follow-up (column 5). As ▶ Table 17.1 indicates, cysts in group 2 are already undergoing involution; it is uncertain whether they are still active and only follow-ups can determine this. Group 3 cysts, on the other hand, are usually inactive because they consist mainly of degenerated forms. The subdivision of cysts into three groups is useful both for planning treatment and evaluating response.
17.1 Echinococci: Types and Epidemiology
17.1.1 Echinococcus granulosus
17.1.2 Echinococcus multilocularis
17.2 Clinical Manifestations
17.3 Diagnosis
17.3.1 Three Main Diagnostic Criteria
17.3.2 Laboratory Parameters
17.3.3 Serologic and Molecular Biologic Tests
17.4 Imaging Studies, Staging of Disease
17.4.1 Historical Background
17.4.2 Morphologic and Functional Classification Systems
17.4.3 WHO Classification
Expanded classifications
WHO stages in the 2001 classification1,3
Clinical classification
Gharbi 19819
Perdomo 198811
Caremani 19978,14
Type I
Type 1 a
CL
Univesicular cysts
Group 1
Active group
Growing cysts
Type 1 a, b, c
Type 2 a
Type I b
Type III a
CE 1
Hydatid sand, double line sign
Type III
Type 3
Type II a, b
CE 2
Multivesicular cyst, rosette or honeycomb pattern
Group 2
Involutional stage
Viable protoscolices likely present
Type II
Type 2 b
Type III b
CE 3
Univesicular cysts with detached endocyst, “water lily” sign from floating membrane
Type III
Type 4
Type IV
Multivesicular cyst with signs of solid transformation
Type IV
Type 5 a, b, c
Type V a, b
Type VI
CE 4
Heterogeneous echo pattern with solid cyst contents; no evidence of daughter cysts
Group 3
Inactive group
Degenerated cyst showing partial or complete calcification; (probably) no viable protoscolices
Type V
Type 6 a, b
Type VII a, b
CE 5
Partial to complete calcification
Group 1: Active Cysts
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree