M. Imran Chaudry • Alejandro Spiotta • Raymond Turner • Harris Hawk • Jonathan R. Lena • Aquilla Turk
The International Symptomatic Aneurysm Trial (ISAT) and the Barrow Ruptured Aneurysm Trial (BRAT) firmly established endovascular therapy as a primary method for treating ruptured intracranial aneurysms. The development of new techniques has broadened the scope of practice to allow for the treatment of geometrically complex aneurysms.1–6 Critical advances in coil technology and the development of novel flexible guide and intermediate catheters providing improved device stability and deliverability, intracranial stents, as well as the introduction of balloon remodeling and flow-diverting stent technology have expanded the indications of endovascular therapy to both wide-necked and fusiform aneurysms. The adoption of these devices has contributed to a larger proportion of aneurysms treated by endovascular means.7
DEVICE DESCRIPTIONS
Distal Access Guide Catheters
Fundamental to any endovascular intervention is the need for stable access to the target lesion. Proximal vessel tortuosity may limit the ability to deliver the guide catheter distally in the vessel of interest, thereby resulting in long distances that the working microcatheter must cover before addressing the target lesion. If the microcatheter and wire travel over redundant segments of a vessel or multiple acute-angled turns, the microcatheter and wire may respond unpredictably when manipulated. Erratic behavior of the microcatheter or microwire may result in technical complications, for example, aneurysm perforation during access for coil embolization and suboptimal stent placement during unsheathing if the microcatheter sails in either direction. The development of neurospecific guiding catheters has resulted in various access devices that offer various advantages with regard to trackability, distal and proximal support, and improved distal access. There is considerable crossover in the design of intermediary catheters and newer generation neurospecific guide catheters. The Neuron 0.053-in and 0.070-in in guide catheters, the 0.088-in Neuron Max sheath (Penumbra, Inc., Alameda, California), as well as the Chaperon 0.071-in guide catheter (MicroVention, Tustin, California) are designed to provide distal guide catheter access, often to the cavernous segment of the internal carotid artery, providing a large stable conduit for intracranial procedures (Fig. 14.1).
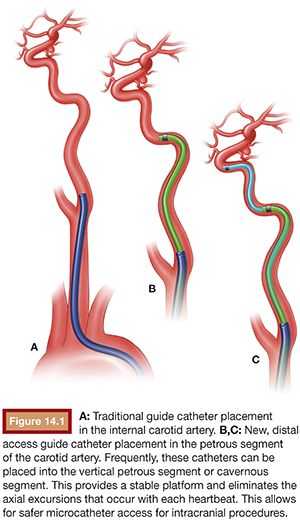
These devices have been shown to be safer than traditional axial guide catheters while providing distal access, often obviating the need for an intermediate catheter. The goal is to place the diagnostic insert into the petrous segment over a 0.038-in hydrophilic guidewire (Glidewire; Terumo Interventional Systems, Somerset, New Jersey) and then advance the guide or sheath over the diagnostic insert into at least the petrous carotid. These larger guide catheters and sheath allow for multiple devices (either balloon and coiling microcatheter or stent delivery catheter and coiling microcatheter) to be deployed simultaneously. The use of larger sheaths may allow for three devices to be deployed simultaneously when coiling with the aid of two balloons, for example.
Microcatheters and Microwires
Endosaccular coil embolization relies on the ability to safely access the aneurysmal sac with a microcatheter that can house a coil. Access to the aneurysm is safest with the microcatheter tracking over a microguidewire. Catheter and microguidewire technology has not significantly changed since the introduction of the 0.0165-in SL-10 (Boston Scientific Corporation, Natick, Massachusetts) microcatheter. To date, the SL-10 remains our workhorse microcatheter as do the 0.014-in Transend Platinum (Stryker Corporation, Kalamazoo, Michigan) and Synchro-2 microwires (Boston Scientific Corporation, Natick, Massachusetts). These two wires deliver the adequate “softness” needed to negotiate tortuous anatomy without causing microperforations while providing sufficient support for over-the-wire microcatheter navigation. Note that intermittent reshaping of the microwire may be necessary during a procedure to navigate a patient’s specific anatomy. We have found that the SL-10 microcatheter tracks favorably over an 0.014-in microwire even in acutely angled vessels. Microcatheters are available in straight and preshaped angled or curved variations, allowing more versatility. Slightly stiffer microcatheters such as the Echelon 10 (Covidien, Irvine, California) maybe used when there is concern for catheter prolapse out of the target aneurysm or when more support for coiling is required. Once access to the aneurysm is obtained, these flexible microcatheters will respond to the stress applied to it by superimposed curves by adopting the parent vessel curvature, which leads to their stability at the aneurysm during coil deployment. The introduction of large-volume coils (Penumbra PC 400; Penumbra, Inc., Alameda, California) has required the introduction of larger 0.025-in coiling microcatheters (Penumbra PX400 and Penumbra SLIM) to allow delivery of these coils. Catheter technology has improved to make these larger and inherently stiffer catheters extremely navigable. However, the main issue with these larger catheters is catching on the lip of the aneurysm neck that may limit catheter access. This may be overcome by advancing the microwire well into the aneurysm, loading the microcatheter and spinning the wire so as to raise the microcatheter from the aneurysm neck. Care must be taken not to load the microcatheter to a degree where it jumps forward into the aneurysm.
Coil Technology
The introduction of coils into an aneurysm provides a thrombogenic mechanical barrier to aneurysmal inflow, which immediately protects the dome while promoting intra-aneurysmal progressive thrombosis and occlusion. The coils furthermore provide a biologic scaffold to promote endothelial growth across the aneurysm neck and vessel healing, resulting in complete exclusion of the aneurysm from the circulation. The initial experience with aneurysm coil embolization did not involve any adjunctive devices such as balloons or stents (“unassisted” coil embolization). As such, the only factors that could be modified by the operator to achieve a successful embolization were the position of the microcatheter relative to the aneurysm neck and dome as well as the size, length, and shape of the coil selected. The introduction of complex coils that could adopt various geometric shapes once deployed resulted in a greater number of aneurysm morphologies considered suitable for coil embolization. The first commercially available detachable coils8 were stiff by today’s standards. Although the duration required for detachment ranged between 10–45 minutes, the detachment mechanism allowed for controlled deployment, repositioning, and removal. This was a significant improvement over detachable balloons used for vessel sacrifice. However, coil distribution of this two-dimensional design (helix) did not consistently allow for good neck coverage. The introduction of coils that adopt an intrinsic three-dimensional (3-D) spherical shape once deployed revolutionized the approach to coil embolization. These would become known as framing coils, thereby creating a robust frame covering the neck and dome of an aneurysm (Fig. 14.2).
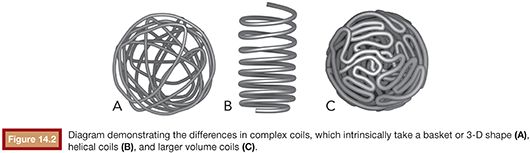
Subsequently, the frame is filled with softer (filling) coils in a “Russian doll” manner. This technique allows for a greater safety margin as the framing coil restricts outward movement of subsequent coils away from the dome and parent vessel and directs filling towards the center of the aneurysm sac. Smaller and softer coils with irregular breakpoints were then introduced to act as “finishing coils” to fill in irregularly shaped neck remnants. The material of the coil greatly affects how it will behave in vivo. Although platinum, which is an inert metal with material characteristics favorable for coil manufacturing, has emerged as the only material in clinical use, variations of coil composition have been introduced to impart additional biologic advantages. For example, a proinflammatory bioactive coil (Matrix; Boston Scientific Corporation, Natick, Massachusetts; Cerecyte; Micrus Endovascular Corporation, San Jose, California) may promote the wound healing cascade and cellular ingrowth into the coil mass and at the neck, whereas a gel–platinum hybrid coil (HydroCoil/HydroSoft; Terumo Interventional Systems, Somerset, New Jersey) that swells to a greater volume when in an aqueous solution such as blood may provide a higher packing density and thereby a lower recurrence rate. Recently, the Penumbra 400 (Penumbra, Inc., Alameda, California) represents another iteration of coil evolution. The Penumbra 400 has a much larger 0.020-in cross-sectional area, which is four times the cross-sectional area of a 0.010-in coil system. By using a nitinol core wire and an outer shell of platinum, the approach was to use a very high volume to length coil to rapidly fill aneurysms. Whatever coil system and strategy is employed, one factor remains constant: results from the Cerebral Aneurysm Rerupture After Treatment (CARAT) study9 support aggressive coil embolization (packing density) as the likelihood of recurrence is related to the degree of initial occlusion.
Balloons
There are five commercially available neurovascular balloons. These are the HyperForm, HyperGlide (Covidien/EV3; Irvine, California), Scepter C, Scepter XC (Terumo/MicroVention, Inc., Tustin, California), and the Transform (Stryker Corporation, Kalamazoo, Michigan) balloons. The HyperGlide, Scepter C, and Transform balloons are compliant balloons intended for the treatment of sidewall aneurysms, whereas the HyperForm and Scepter XC are hypercompliant balloons intended for use in the treatment of wide-necked bifurcation aneurysms. These hypercompliant balloons tend to protrude well into the aneurysm neck and may be used to preserve vessels that arise within the neck of an aneurysm. To a large degree, we have found the compliance of the balloons to be somewhat similar and the balloons maybe used interchangeably. What sets the balloons apart is their ability to navigate tortuous, difficult anatomy as well as their stability at the aneurysm neck.
Stents
Currently, there are two commercially available nitinol self-expanding stents: an open-cell Neuroform stent (Stryker Corporation, Kalamazoo, Michigan) and a closed-cell Enterprise Vascular Reconstruction Device (VRD) (Cordis Corporation, Miami Lakes, Florida). Briefly, the open-cell design may have better wall apposition around sharp turns; however, it may create a shelflike extension into an aneurysm that arises around an acute bend or bifurcation (basilar apex). This shelf may be advantageous depending on its position but has the risk of coil loops prolapsing into the parent vessel. The closed-cell design of the Enterprise VRD may have suboptimal wall adherence around turns, but this can be reduced by more pushing of the stent as it is deployed rather than a true unsheathing. In theory, there is a lower chance of coil loops prolapsing into the parent vessel. On the other hand, newer, self-expanding braided stents are currently available as part of investigational device exemption (IDE) or 510(k) trials and should be commercially available within the next 2 to 3 years. Within the near future, we expect to see the commercial availability of braided stents such as the Low-Profile Visualized Intraluminal Support (LVIS) and LVIS Jr. (Terumo/MicroVention, Tustin, California). The advantage of these self-expanding braided stents is that there is better wall apposition around turns, and the braided structure allows for pore sized such that coil loop prolapse is highly unlikely.
Flow Diversion
Large and giant aneurysms and fusiform aneurysms remain a challenge to treat, whether from an endovascular or open standpoint. The Pipeline Embolization Device (PED) (Covidien/eV3, Irvine, California) is a novel flow-diverting stent that revolutionized the treatment paradigm for large and giant aneurysms from the petrous internal carotid artery to the ophthalmic segment. The PED is a high mesh braided stentlike device that allows for remodeling of the parent vessel wall and gradual occlusion of an aneurysm. The use of these devices may be technically less challenging than traditional treatments (open clipping, coiling, balloon, or stent coiling) of these aneurysms; however, the long-term durability and morbidity/mortality related to the use of these devices remain to be seen. Currently, there is a randomized study (LARGE Aneurysm Randomized Trial: Flow Diversion versus Traditional Endovascular Coiling Therapy) that looks to evaluate the treatment of large and giant aneurysms with flow diversion versus traditional coiling methods. We eagerly await these results.
TECHNIQUES
Unassisted Coiling
The ISAT firmly established endovascular therapy as the primary method for treating ruptured aneurysms. With the considerable advances in coil technology, endovascular therapy is becoming the accepted treatment for ruptured and unruptured aneurysms. The introduction and development of complex framing coils, filling coils, and finishing coils have made many aneurysms previously considered uncoilable amenable to endovascular therapy.
The goal of any “coiling” procedure is the complete occlusion of the target aneurysm and the prevention of aneurysm rupture. The ideal aneurysm for unassisted coiling is one with a favorable neck (<4 mm) or dome-to-neck ratio greater than 2:1. Small-necked aneurysms are much more amenable to primary coiling, using the shoulders of the aneurysm neck to buttress the coils and keep them within the aneurysm sac. In smaller aneurysms (3 to 10 mm), the catheter is ideally positioned at the neck or within the proximal one-third of the base. In larger aneurysms, an “around the world” technique maybe used in which the coiling microcatheter is brought into the aneurysm and the tip placed approximately three-quarters of the way around the aneurysm sac in such a manner that the catheter is essentially pointing back at the neck. This allows the catheter to gradually migrate back to the neck, preventing early kick out of the catheter. Although there are many techniques in framing, filling, and finishing, we tend to use the “Russian doll technique.” We frame with a framing coil with the goal of getting neck coverage and systematically stepping down in coil size to coil within the coil mass. Coil diameters are sized to the largest diameter in spherical aneurysms, and in irregular oblong or bilobed aneurysms, coil diameter size is often based on the average diameter ([Length × Width × Height]/3). Commonly, we will use multiple framing coils (decreasing in diameter and length) to frame and fill the aneurysm and subsequently switch to soft helical or complex finishing coils to pack both the aneurysm sac and neck. Our goal is always complete occlusion of the aneurysm (Fig. 14.3).
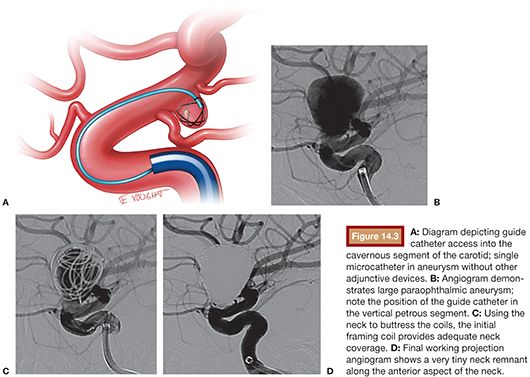
Dual Catheter Technique
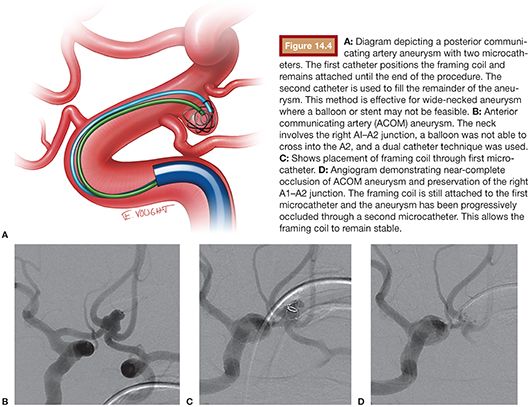
The dual catheter technique was an earlier technique employed in the treatment of irregular wide-necked aneurysms before the availability of balloons and stents. It is still used occasionally in the setting of subarachnoid hemorrhage where stents (and antiplatelet therapy) is contraindicated and in tortuous small vessel anatomy where a balloon may not navigate. In this technique, two microcatheters are introduced into the aneurysm sac. The first is usually positioned at the neck through which a framing coil is delivered. The second catheter is placed into the aneurysm sac through which filling and finishing coils are introduced. The initial framing coil is not detached until the end in attempts to maintain its position and stability throughout the case. Commonly, the framing coil is undersized to prevent encroachment into the parent neck (Fig. 14.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








