Intracranial Infection and Inflammation
Ramesh S. Iyer, MD
LEARNING OBJECTIVES
1. List at least three common congenital infections using TORCH mnemonic.
2. Recognize typical intracranial imaging features of a TORCH infection.
3. Identify meningitis and its extra-axial complications (e.g., subdural empyema) on MR.
4. Distinguish cerebritis from brain abscess on MR.
5. Recognize the typical appearance of viral encephalitis on MR, along with more specific manifestations of herpes and HIV encephalitis.
6. Recognize acute disseminated encephalomyelitis (ADEM) on MR.
INTRODUCTION
Intracranial infections in children are relatively uncommon. When they do occur, they may be life threatening and result in permanent neurologic damage. Urgent medical attention or surgical intervention is required in most cases. Hence, imaging of the CNS plays a central role in diagnosis and efficient triaging of care. CT is usually the initial exam performed. This allows for identifying hydrocephalus, sulcal effacement, hypodense parenchymal edema, and extra-axial collections that may be present. Contrast-enhanced MR is the study of choice for characterizing intracranial infection and its complications. MR venography should also be performed if associated sinovenous thrombosis is suspected.
TORCH INFECTIONS
The brain is susceptible to infection in utero because both the CNS and the immune system are rapidly developing during fetal life. “TORCH” is a mnemonic for some of the more common congenital infections:
T: Toxoplasma gondii
O: Other (for instance, human immunodeficiency virus [HIV], syphilis)
R: Rubella
C: Cytomegalovirus (CMV)
H: Herpes simplex virus 2 (HSV-2)
Most infections are transmitted to the fetus from transplacental passage, though transvaginal transmission during delivery is also possible. As a general rule, infections acquired during the first two trimesters result in congenital brain malformations (please refer to Chapter 30). Those occurring during the third trimester tend to result in destructive brain lesions.1
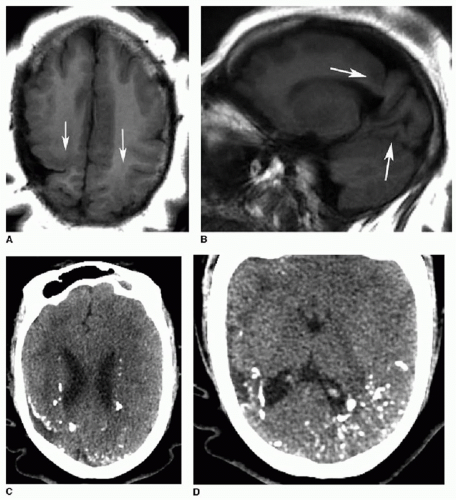
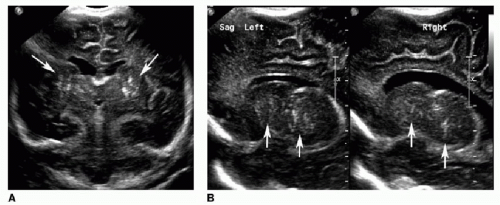
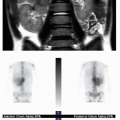
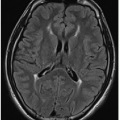
Cytomegalovirus is the most common TORCH infection, with up to 4,000 infants born annually in the United States with symptomatic congenital CMV infection. One percent of all live births show evidence of in utero CMV infection, but the majority of these are asymptomatic. Clinical manifestations of CMV infections include microcephaly, chorioretinitis, sensorineural hearing loss, skin rash, and hepatosplenomegaly. Cross-sectional imaging findings include parenchymal calcifications with a predominantly periventricular distribution, ventriculomegaly, prominent cerebral sulci, and periventricular and subcortical white matter density or signal changes (Fig. 33.1). Early CMV in utero infection is associated with neuronal migrational anomalies, for example, polymicrogyria, schizencephaly, and lissencephaly (Fig. 33.2). On cranial US, the basal ganglia and thalami may exhibit branching curvilinear hyperechogenicities called lenticulostriate vasculopathy (Fig. 33.3). This is a nonspecific sonographic finding that may be seen in other pathologies including toxic or ischemic insults, congenital heart disease, and trisomies 13 and 21.1, 2 and 3
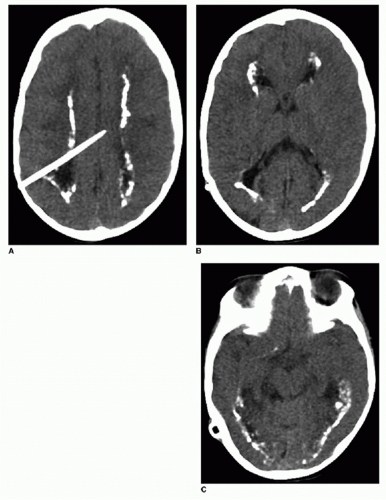
Congenital toxoplasmosis, or in utero infection with T. gondii, is the second most common TORCH infection. Domestic cats are the major source of toxoplasma infections in humans. Common clinical features are chorioretinitis, seizures, and hepatosplenomegaly. Imaging findings of toxoplasmosis include ventriculomegaly, encephalomalacia, and parenchymal calcifications (Figs. 33.4 and 33.5). The calcifications of toxoplasmosis are often more widespread—basal ganglia, periventricular, deep, and subcortical white matter—than are those in CMV, which tend to cluster around the ventricles.1, 2 and 3
ACUTE BACTERIAL INFECTIONS
Meningitis and its complications
Bacterial meningitis is the most common form of pediatric CNS infection. It is most often caused by hematogenous spread from another site of infection, such as the respiratory tract. Meningitis may also be caused by contiguous spread from the sinuses and mastoids.2 In immunocompetent children and adolescents, the most frequent causative organisms are Streptococcus pneumoniae, Neisseria meningitides, and Haemophilus influenzae type b (Hib). There is a wider variety of offending pathogens in infants, notably group B Streptococcus.1
Uncomplicated meningitis, which is suspected clinically and confirmed by laboratory assessment of cerebrospinal fluid obtained by lumbar puncture, often yields normal CT and MR examinations. While brain imaging has a low sensitivity for identifying meningitis, it is useful in detecting complications of meningitis. Contrast-enhanced studies may show leptomeningeal enhancement, with postcontrast MR more sensitive than CT (Fig. 33.6A, B). There may also be FLAIR high signal in the subarachnoid spaces surrounding the brainstem and within the cerebral sulci on MR. In bacterial meningitis, there is typically leptomeningeal enhancement over the cerebral convexities. In granulomatous meningitis, including tuberculosis, there is more often T2-weighted/FLAIR high signal and enhancement in the basal cisterns.1,3
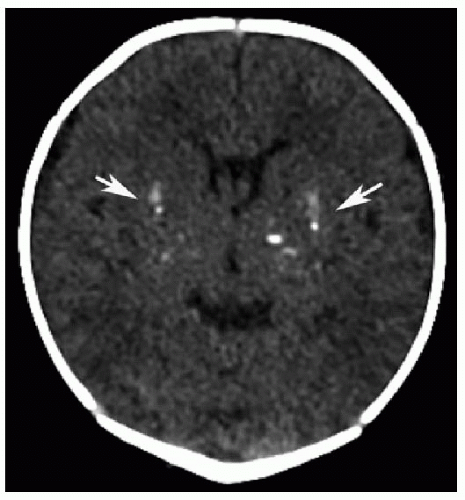 FIG. 33.4 • Congenital toxoplasmosis in a 1-week-old male. Axial noncontrast CT image exhibits bilateral basal ganglia calcifications (arrows). |
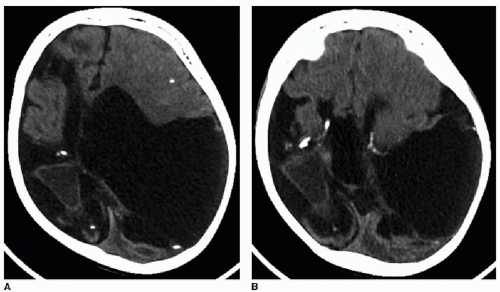
The complications of meningitis are best evaluated with MR. Ventriculitis occurs when the infection spreads to the ventricular system, often through the choroid plexus. There is enhancement of the ependymal lining, ventriculomegaly, and diffusion restriction of the ventricular fluid. Subdural empyema is a purulent infection in the subdural space. These extra-axial collections are hyperintense to CSF on FLAIR and exhibit peripheral enhancement and diffusion restriction, similar to an abscess (Fig. 33.7). When such a collection does not restrict diffusion, the term subdural effusion is used. Subdural empyemas are generally treated with operative drainage, while effusions are managed conservatively.1,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree