Fig. 41.1
The probe is moved over the region of interest, while each of the 100–200 images is tagged with position data from the optical tracking camera and reconstructed into a regular 3D volume (a). The pointer steers the display of the 3D volume (b)
Intraoperative 3D US can be used alone for navigation, but it is often useful to register 3D MR volumes to the patient’s head before start of the operation as in a conventional navigation system. The use of preoperative data will make it easier to plan the approach and craniotomy; it will facilitate ultrasound image orientation and help the less experienced US user to interpret the US images.
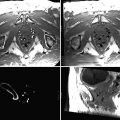
In addition to tissue images, it is also possible to make 3D images of vessels (US angiography) based on recordings of the power Doppler signals from the bloodstream. In the intraoperative imaging system that we use, the vessels are presented as red overlay in the tissue images (Fig. 41.2).
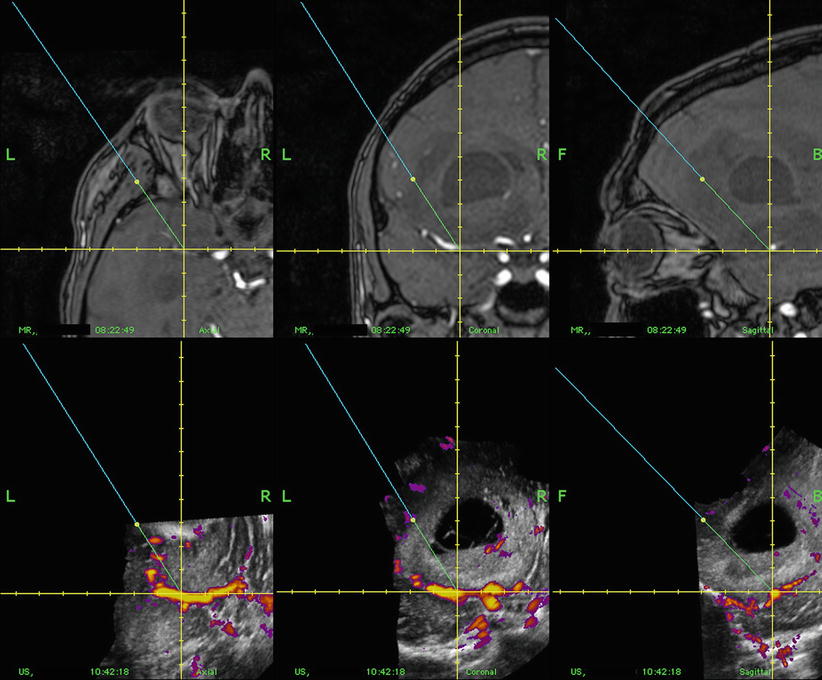

Fig. 41.2
Corresponding cross sections of 3D MR (upper row) and 3D US (lower row) in orthogonal display (axial, coronal and sagittal plane). The 3D US system enables concomitant acquirement of both tissue signals and power Doppler angiography. The patient had a glioblastoma
The US volumes can be acquired when needed during the operation, which ensures that navigation of the instruments is performed on updated US image maps throughout surgery. Furthermore, the transducer probe is not in the operation field during the operation, except when new 3D US image acquisitions are being acquired.
Display of the Images
When preoperative MR or CT is used, the 3D US data can be visualized side by side to the preoperative data or fused with the preoperative data. The display modalities offered by the ultrasound-based navigation system are orthogonal slicing (Fig. 41.2), anyplane as well as dual anyplane slicing (Fig. 41.3), and stereoscopic projections. The stereoscopic display has been used for visualizing vessels.
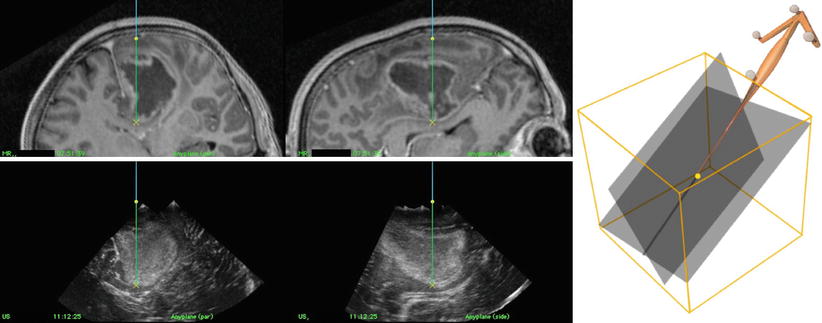

Fig. 41.3
Corresponding cross section of preoperative 3D MR (upper row) and intraoperative 3D US (lower row) of a glioblastoma in dual anyplane – a plane defined by the direction and rotation of the pointer (anyplane) + a plane perpendicular to the anyplane
The slicing and display of the volumes on the monitor is steered by a pointer (Fig. 41.1b) or another tracked instrument like the CUSA, the biopsy forceps, an endoscope or a microscope.
Navigation Inaccuracy
The overall clinical inaccuracy is the difference between the position of an object in the true physical space and the position of the object as displayed in the image space. When using preoperative images (MR or CT), the overall clinical inaccuracy consists of both registration inaccuracy, describing the inaccuracy of the registration of the images to the head, technical inaccuracy, indicating the inaccuracy in the navigation system itself, and the inaccuracy due to brain shift. The registration inaccuracy can be considerable when using fiducial markers. The registration inaccuracy of MR and CT is even larger with landmark registration [23]. For 3D US, the registration inaccuracy is eliminated because both US acquisition and navigation based on 3D ultrasound are done in the same reference system. The inaccuracy due to brain shift can mostly be abolished by repeated acquisitions of 3D US during the operation. The overall clinical navigation system inaccuracy of the US-based navigation system SonoWand Invite® is less than 1.5 mm [24].
Image Quality
Image quality is of crucial importance in intraoperative imaging. The US image quality is related to the spatial resolution (the ability to resolve targets), which in the 2D US scan plane is depending on the frequency of the probe used. Higher frequency means better axial resolution. The drawback is increased attenuation and thus loss of penetration in the tissue with higher frequency. Further, the size of the probe footprint, i.e. the aperture of the transducer array, is an important factor for the lateral image resolution. Generally, a larger probe footprint means higher lateral resolution.
One of the reasons why US image quality has improved dramatically in recent years is that the probes can be electronically tuned for a range of frequencies. In this way optimal resolutions can be obtained simultaneously at multiple depths, resulting in high image quality at a broad range of depths. Also, ultrasound elements can be made smaller and smaller, making it possible to make slimmer high-resolution transducer probes. The images generated by a phased-array probe spread out like a fan. It will cover a wide area at a distance from the probe, but close to the probe it has a small sector. Such probes (4–8 MHz) are suitable for deep-seated lesions. A drawback with phased-array technology is the inability to display tissue adjacent to the probe, i.e. superficial to the probe’s focal point. To overcome that problem, a plate of gelatine, about 1 cm thick, is formed and placed on the brain over the area of interest. Since gelatine does not generate any echo at all, it only lifts the probe away from the lesion, optimizing the distance between a superficial lesion and the probe.
For lesions close to the surface, a flat linear array probe will in most cases be more suitable. The probe generates a rectangular image limited by its footprint in width, but image quality can be extremely good depending on the aperture and frequency of the probe.
Clinical Aspects of Obtaining Good Image Quality
Less experienced users of US imaging technology have sometimes been unable to achieve good image quality because they have not paid enough attention to the nature of US penetration in tissue. Air located between the probe and the dura or in the operation channel will give acoustic shadows and poor image quality. Sterile gel may be applied between the probe and the dura to ensure good beam penetration. Sometimes, calcifications in the dura may produce acoustic shadows and reduced image quality, which may be overcome by simply acquiring new US images after the dura is opened. When the images are acquired over bare brain, water will provide sufficient acoustical contact. Some probes may be sterilized, but still most probes need to be wrapped in sterile drapes before use. When packing the probe before the operation, it is important to apply gel on the probe head in the sterile plastic drape to remove air and thus obtaining optimal conditions for beam propagation.
To be able to record new US volumes with good image quality throughout the operation, the positioning of the patient is important [25]. The patient should be positioned so that the preferred operation channel becomes vertical (Fig. 41.4a). Thus air bubbles will rise to the surface when the operation cavity is filled with saline for 3D US acquisition, which is important in order to avoid air artefacts. Such a positioning is always possible (own experience). The most challenging approaches are immediately in front and immediately behind the central sulcus. In the former cases we use a supine position with the neck flexed. Sometimes it is necessary to raise the angle of the head end of the operating table during image acquisition. For lesions behind the central sulcus, we use prone or park bench positions. Park bench is a very flexible and useful position that should be coped within any operation theatre. Even though the surgical access turned out to be oblique, it is often possible to achieve a vertical access during image acquisition by adjusting the table, and then turn it back to continue the operation.
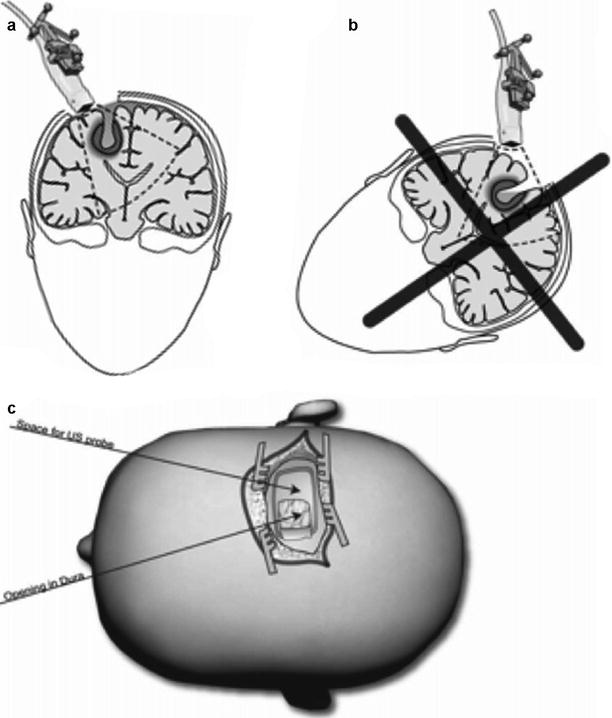

Fig. 41.4
The patient should be positioned to obtain a vertical access to the lesion (a). In that way it is possible to fill the operation cavity with water for renewed 3D US acquisition. In a non-vertical access it will be difficult to acquire a 3D UD volume with good image quality (b). In deep-seated lesions a linear incision of the skin and an ellipsoid craniotomy are beneficial (c). Image at an angel to the operation access may be useful to reduce artefacts from the operation channel
By these small modifications of standard positions, it is usually possible to get good images throughout the operation. The only obvious exception is operations in sitting position.
Even though only a small craniotomy is needed for 3D US-guided surgery on deep-seated lesions, it is often useful to make an ellipsoidal or rectangular craniotomy (Fig. 41.4c), to be able to acquire images at an angel to the surgical channel. This reduces the artefacts from the operation channel.
A vertical access will also reduce the retraction on the normal tissue because the retractors do not have to work against gravity. Retractors in the surgical cavity should generally be removed before image acquisition to reduce artefacts and shadows. The image quality during the operation is also dependent on having a clean resection cavity. Blood clots, cottonoids, etc. must be removed, and the cavity should be flushed with saline to remove air bubbles and tissue particles before 3D US acquisition. The preparation of the resection cavity for a new ultrasound acquisition may take a few minutes, depending on how clean the cavity is during the operation. The process of acquiring and processing new images takes about 10 s. Thus, several ultrasound acquisitions will not alter the workflow of the operation to any significant degree.
3D US in Everyday Neurosurgery
Intraoperative navigated 3D US has been used for a wide variety of applications. At our department we use it for 95 % of intra-axial brain tumour resections [26], for all biopsies and for all cavernomas and AVMs [27]. We also use it for pituitary tumours [28], skull base cases and sometimes to guide endoscopes [29] as well as insertion of ventricle catheters.
Tumour Surgery
3D Ultrasound-Guided Tumour Resection
3D MR may be used for the planning of optimal positioning of the patient and optimal bone flap. After the craniotomy, guiding of the operation must be based on the 3D US volume as it gives a more correct image of the intraoperative situation.
We very often choose a transcortical approach. With very small, self-holding spatulas, we make the shortest possible approach though non-eloquent cortex and white matter. The approach through brain should be carefully planned based on the 3D US volume to avoid too much expansion of the channel in normal tissue during the surgery.
The information in the 3D US volume can be explored in a conventional way by using a pointer. The drawback is that the operation has to be stopped while pointing in the resection cavity. A more elegant and dynamic approach is to attach an optical tracking frame to the resection instrument (e.g. CUSA). In that way it is possible to continuously follow the position of the resection instrument during the operation (Fig. 41.5). While the conventional axial, sagittal and coronal display is frequently used for planning, our experience is that slicing controlled by the position and orientation of the operation instrument (anyplane) is more useful when guiding the resection. Another image plane perpendicular to this plane (dual anyplane) gives additional value especially when working close to tumour borders where one plane could be tangential to the tumour. The monitor has to be located so that the surgeon only needs to glance to the side of the microscope in order to see the navigation display.
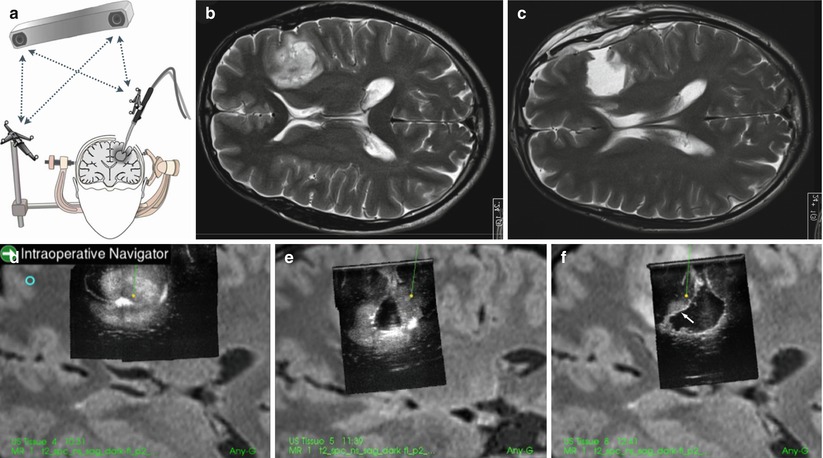

Fig. 41.5
With tracking of the ultrasound aspirator (a), the position of the tip of the instrument can be continuously monitored in the 3D US volumes (d–f) of a low-grade glioma. In (f) there is still some remaining glioma tissue (indicated with an arrow) that was removed guided by the navigated ultrasound aspirator. In (e, f) there is a considerable shift of the surface of the brain. (b) shows the pre-op MR and (c) shows the MR 1 day after the operation. Gross total resection was achieved
To work with guided instruments is especially useful in deep-seated tumours. The resection can be done through a very small opening with visual information from the microscope and position information from the navigated 3D US volume. This will reduce the injury to normal tissue. The on-line information about the distance from the tip of the resection instrument to the tumour borders, or to important vessels, may speed up the resection and make the surgeon feel more confident in different phases of the operation. It is necessary, especially in large tumours, to acquire several new 3D US volumes during the operation, to have as updated and correct tissue information as possible.
3D US-Guided Surgery in Eloquent Areas
Surgery in eloquent areas represents a special challenge. Intraoperative stimulation in wake patients is considered a gold standard for localization of eloquent brain function, but it is time consuming, troublesome for the patients and prolongs surgery. Our solution is to import MRI volumes with overlaid outline of fMRI and diffusion tensor tractography (DTT) data into the 3D US-based imaging system, SonoWand® [30, 31]. This system allows concomitant display of both the MRI volume with overlaid fMRI/DTT and the 3D US. By virtually extending the tip of the pointer displayed in the system, we can easily see possible registration inaccuracies of the MRI by pointing at anatomic landmarks or tumour borders in the 3D US. By the pointer (with virtual off set) we can also localize the relative position of fMRI and DTT outline in the 3D US.
In our experience, the fMRI and DTT areas usually lie outside the tumour margins visualized by ultrasound in patients without neurological deficits (Fig. 41.6). The resections can therefore be safely performed according to these tumour margins in the 3D ultrasound images, with particular care in areas neighbouring eloquent areas. This only requires the surgeon to do repeated 3D US acquisition during the operation to correct for brain shift and then carefully resect the tumour within the ultrasound visible borders displayed on the navigation system. A navigated operation instrument (CUSA) makes it much easier to perform the operation.
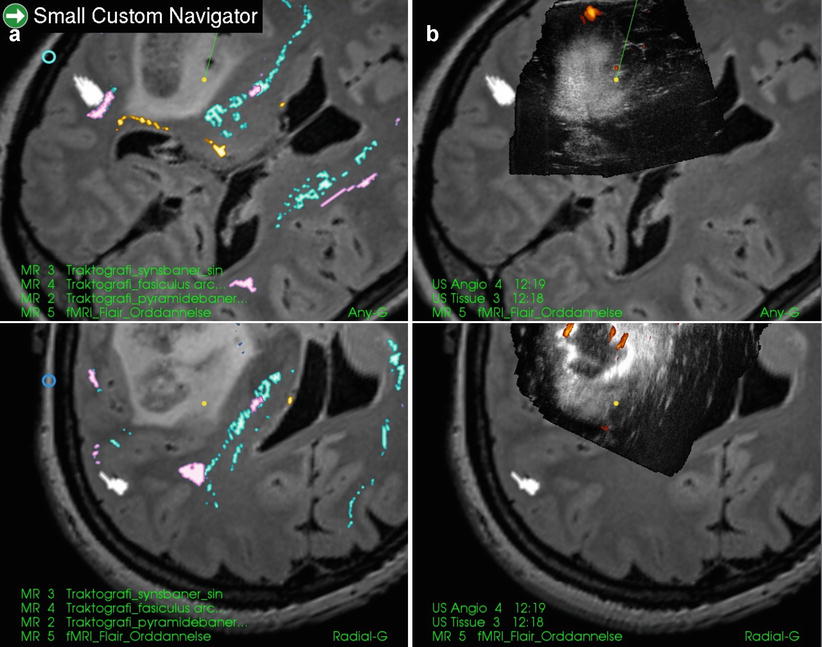

Fig. 41.6
An insular glioma (anaplastic astrocytoma) with fMRI and tractography displayed in anyplane (upper row). The lower row is a plane perpendicular at the tip of the pointer (tip plane). The areas of arcuate fasciculus (pink), pyramidal tract (blue), optical tract (yellow) and fMRI of speech (white) are all located outside the tumour as shown in preoperative 3D MR and 3D US. In 3D US some tumour has already been removed
With this system gross total resection (GTR) was obtained in 32 % of glioma operations in eloquent areas. Only 3 % of the patients had a neurological deterioration due to injury of eloquent tissue. When the lesion to eloquent area distance was less than 5 mm, 24 % underwent GTR and 10 % experienced neurological deterioration [32].
High-Grade Gliomas
Anaplastic gliomas and glioblastomas are easily depicted with 3D US. A large oedema may give some signal in the US image, but it very seldom represents a challenge in 3D US-guided surgery. There is a difference in signal intensity between oedema and tumour tissue. Also, most patients have been using steroids for at least a week before surgery to reduce oedema. A biopsy study where we compared image interpretation with histology of navigated biopsies has been performed [33]. We found that only 2 % of biopsies collected in a 2–7 mm range inside the 3D US depicted tumour border were normal tissue (false positive). At the same time we found that 10 % of the biopsies collected in a 2–7 mm range outside the depicted tumour border were tumour (false negative).
Gross total resection (GTR) is associated with improved survival of high-grade gliomas [34]. The gold standard for GTR is total removal of contrast-enhancing tissue in high-field postoperative MRI. Resection grade can be studied in ideal conditions to test the efficacy of an intervention, such as in highly selected trials. However, results from highly selected cases may not be of relevance to the average patient. Effectiveness means how it works in real life in nonideal circumstances, with unselected patients and different, unselected surgeons. We have done a study to test the effectiveness of 3D US-guided resection in high-grade gliomas [26]. In a subgroup with the same inclusion criteria as in the 5-ALA study [35] and found a comparative GTR of 63 %.
The modified ranking scale before and after surgery gave an indirect indication of the value of 3D US in avoiding complications during surgery. Functional deterioration was seen in only 6 % of the cases where ultrasound image quality was good or medium but in 45 % of cases where the image quality was poor [26].
High-grade gliomas in eloquent area can be operated guided by fMRI and tractography imported into the SonoWand system and based on 3D US, as described above. In our material median survival of glioblastoma patients with lesions in eloquent area was 13.2 months [32].
Obtaining GTR has a modest effect on survival of the glioblastoma patients. The quality of life (QoL) therefore become of paramount importance. In a prospective study of the effect of QoL of operation of glioblastoma patients, we found that some patients improved and others impaired in QoL after surgery [36]. Factors that were independently associated with loss of quality of life were new neurological deficits and occipital lesions. In addition it was found that no use of 3D US was an independent predictor for worsening of quality of life. One explanation could be that active use of 3D US will lead to less harm also on non-eloquent normal tissue, which could influence the postoperative quality of life.
Low-Grade Gliomas
US is an excellent imaging modality to depict low-grade gliomas. There is no oedema in low-grade gliomas, so the lesion seen in the images is tumour tissue. A study where biopsies were collected inside and outside the imaged tumour border with a navigated biopsy forceps revealed that US is at least as precise as MR T2 in delineating the tumour border [37]. As expected some biopsies taken between 2 and 7 mm outside the imaged border contained tumour cells (false negative), but no biopsies collected from 2 to 7 mm inside the imaged tumour border was normal (no false positive). Accordingly, navigated US usually depicts the borders of low-grade gliomas in a very safe way. There are, however, some rare cases where the tumour signal is weak both in MR and 3D US. In those cases it might be dangerous to do resection because of the risk to harm functioning brain tissue.
Resection of Low-Grade Gliomas in Eloquent Areas
As described in a previous paragraph, MRI with overlaid fMRI and DTT can be imported into the 3D US imaging system [30]. The location of eloquent cortex and the tracts in the 3D MRI cannot be trusted due to registration inaccuracy and brain shift. Several research groups are working on automatic methods for updating preoperative MR images using 3D US. In our experience low-grade gliomas in eloquent areas can be handled with only 3D US, because in patients with normal function, both eloquent cortex and tracts seem to be located outside the depicted tumour (Fig. 41.6). It is, however, important to be careful and acquire new 3D US volumes several times during the operation to be able to operate on an updated image
Skull Base Tumours
In skull base tumours navigation based on preoperative MR is often inaccurate, mainly due to inaccurate registration. 3D US is useful to ensure correct navigation. In large skull base meningiomas or very large acoustic neurinomas, 3D US-guided resection can be used to do a safe and rapid subcapsular resection without having to do any dissection of the capsule [38]. It is much easier to peel the capsule off the normal brain or brainstem when most of the tumour is removed for two reasons: first, a lot of space in the operation field makes it easier to dissect, and second, a thin, flexible capsule comes off more easily than a stiff capsule filled with tumour tissue.
In some large tumours important normal vessels, that should not be injured, may be found inside the tumour. The display of the US angiography will show the distance between the instrument tip and the vessels (Fig. 41.7). During resection there might be a shift of these vessels. Therefore it is important to acquire new 3D US volumes to have reliable images. In vascular meningiomas with deep-seated blood supply, the 3D US angiography will show the localization of these vessels, and a rapid trans-tumour approach and coagulation of these vessels will reduce the total blood loss of the operation and make it easier to perform.
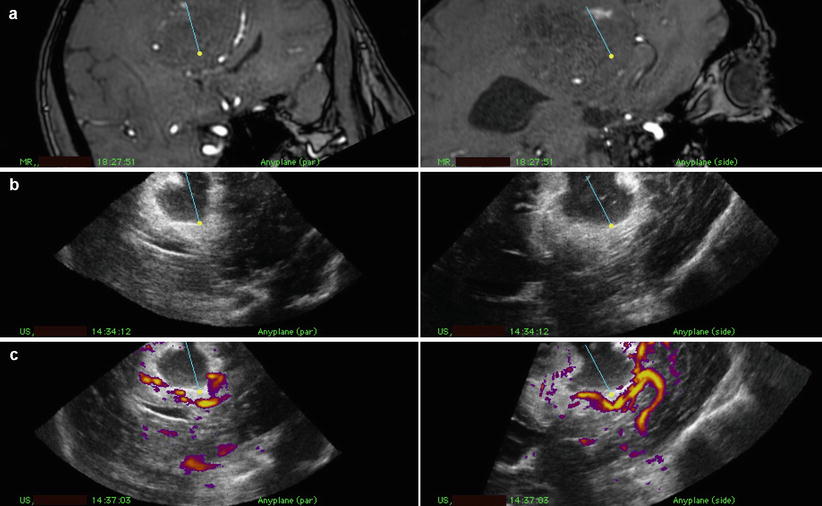

Fig. 41.7




Snapshot from the navigation monitor during operation for a gigantic parasagittal meningioma in dual anyplane with preoperative MRA (a), 3D US tissue after resection of a larger part of the tumour with a central resection cavity (b) and 3D US angiography (c). The pericallosal artery is seen close to the indicator of the resection instrument (CUSA)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree