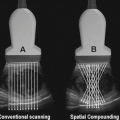
Fig. 15.1
Ultrasound probes used in urology . (a) Linear array (b) section/vector (c) curved array (d) laparoscopic (e) transrectal biplane (f) robotic ultrasound probe
Linear: The linear array scanners produce sound waves parallel to each other which generate a rectangular image. The width of the image and number of scan lines are the same at all tissue levels. Thus coupled with high-frequency settings, this probe has great near-field resolution. The linear transducer can be used for viewing surface texture of the liver. The disadvantage of this probe is the tendency for artifacts when applied to a curved part of the body which generate air gaps between skin and transducer.
Sector/vector: It produces a fanlike image that is narrow near the transducer and increases in width with deeper penetration. It is useful when scanning between the ribs as it fits in the intercostal space. The disadvantage is poor near-field resolution.
Curved: The curved probe is a compromise of the linear and sector scanners. The density of the scan lines decreases with greater distance from the transducer, but not to the level as sector scanners. Often imaging requires greater depth of the acoustic waves, so lower frequencies (3–5 MHz) are used. This lower frequency also allows scanning for patients with various body habitus. The curved probe may be difficult to use in curved regions of the body, e.g., the spleen behind the left costal margin.
Laparoscopic ultrasound (LUS): Laparoscopy with the assistance of ultrasound avoids unnecessary open surgery and improves selection of patients for renal and adrenal tumor resection. The diameter is less than 10 mm to allow introduction through a 10–11-mm laparoscopic port. The length is typically 35–50 cm and with articulating tips to allow imaging to any location in the abdominal cavity. LUS enables direct contact of the probe with the target tissue thus enabling the use of high-frequency (6–10 MHz) waves to improve resolution with a depth of 4–10 cm. LUS may be used to identify and characterize tumors, guide biopsy needles and probes, and monitor the freezing zone during cryoablation . Challenges of LUS include limitations of the small working space resulting in images from oblique planes.
Transrectal ultrasound: (a) End fire is a linear array whose direction of maximum radiation is along the axis of the array; it may be either unidirectional or bidirectional; the elements of the array are parallel and in the same plane, as in a fishbone antenna. (b) Biplane is composed of two arrays: one linear for imaging of the longitudinal plane and a highly curved one to image the transverse plane. These two planes allow simultaneous visualization of perpendicular planes in real time .
The Kidneys
The same principles and techniques to perform renal procedures guided by ultrasonography apply to interventional techniques.
Percutaneous Nephrostomy and Percutaneous Nephrolithotomy
Percutaneous access to the renal collecting system was first described in 1955 by Goodwin et al. as a means to either drain or stent an obstructed urinary system [1]. Beyond the usual indications for an obstructed urinary system, percutaneous nephrostomy (PCN ) can be used to access the collecting system in an antegrade manner for definitive treatment of nephrolithiasis, commonly performed by laser lithotripsy [2]. Generally, for percutaneous approach the left kidney is more challenging than the right due to the rib cage as described previously during the Kidney US chapter. Reports show that only 11 % of urologists perform their own PCN access and majority uses fluoroscopic guidance, but given appropriate training and use of ultrasound guidance, this technique still remains well within the realm of the urologist as seen in some European and Asian countries [3–5].
Ultrasound probe: For adult abdominal scanning, a curved-array transducer 3–5 MHz is used. For pediatric patients, a higher-frequency transducer may be utilized. The use of color Doppler provides good visualization of vascular structures within the kidney, and the probes usually allow attachments that can display the path of the needle towards the target (calyx or stone).
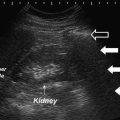
Technique: Hydronephrosis on ultrasound will demonstrate enlarged renal calyces and pelvis that appear black (hypoechoic), indicating the presence of fluid. While acute hydronephrosis generally does not affect renal parenchyma, chronic obstruction of the urinary tract can result in thinning of the renal cortex (atrophy) and blunting of the renal papillae. Renal stones are identified as hyperechoic structure on US with shadowing, and color Doppler can identify vascular structures (Fig. 15.2). Presence of these findings on ultrasound examination should be noted. Additionally, the operator should attempt to visualize the ureter, which may be dilated depending on etiology.
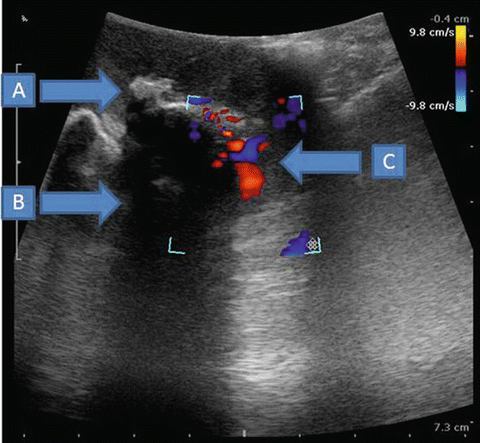

Fig. 15.2
Large hyperechoic renal stone with acoustic shadowing and color Doppler of vessels. (a) Large renal stone (b) acoustic shadowing (c) vessels on color Doppler
There are several described techniques, but the two most common are a one-stab technique and the Seldinger technique for placement of a PCN tube. The one-stab technique is usually reserved for moderately to severely dilated collecting systems. Meanwhile the Seldinger technique can be applied with or without dilation of the tract [6]. Both techniques are performed with the patient in a prone or prone-oblique position (Fig. 15.3). Recently, the supine position has been popularized for percutaneous nephrolithotomy (PCNL ) [7]. The one-punch technique involves the use of ultrasound imaging to guide placement of a hollow 18-gauge needle with a sharp, beveled edge mounted to a pigtail catheter . Once inserted into the hydronephrotic collecting system and a flash of urine is obtained, the catheter can be slid over the needle and the needle subsequently removed. Real-time ultrasound is used at all times to monitor the target and ensure that the needle, wire, and dilators enter the calyx and do not violate other structures (Fig. 15.4). If only drainage is needed, a 12-French PCN tube can then be inserted over the wire with curl confirmation on ultrasound imaging. Imaging can be enhanced by injecting sterile saline to identify the collecting system under US guidance. Further serial dilation or inflating balloon dilators can be performed with dilators up to the size of the instrument sheath (24 °F), ensuring that the wire remains in place for access purposes throughout the operation. In case of staghorn calculi, ultrasound guidance towards the stone or puncture of an alternate calyx may be required. The patient in a prone position allows the US probe to examine from the most lateral side of the abdomen under the rib cage and scan medially to enable a global view of the anatomy. The serious limitation of the ultrasound probe is the two-dimensional view of the kidney, calices, and the path which the needle may traverse injuring a viscera. The risk factors for colonic perforation are the following: the presence of colonic distension due to previous intestinal bypass surgery, female sex, elderly, thin patients, the presence of a horseshoe kidney, and previous renal surgery placing the colon in a more retroperitoneal position. The incidence of colonic injury is also greater on the left side, with a lower caliceal puncture, and with an extreme lateral origin of the percutaneous puncture [8]. Nevertheless, no statistically significant evidence has shown the implication of these factors in the development of colonic injury. Moreover, injuries to the spleen may occur on the left and liver on the right PCN L or PCN.
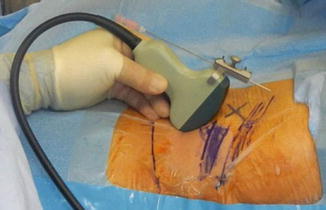
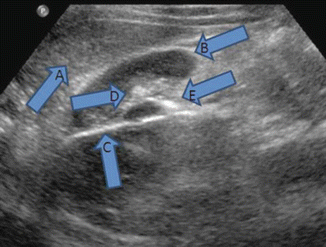

Fig. 15.3
Percutaneous nephrolithotomy with needle-probe attachment. Blue marking of ribs

Fig. 15.4
Transverse view of the right kidney. (a) Liver (b) right kidney (c) percutaneous needle (d) collecting system (e) stone
Clinical data: There has been one randomized trial that compared ultrasound-guided PCNL access to traditional fluoroscopic access and found comparable results with less exposure to ionizing radiation but longer access time (11.0 min vs. 5.5 min) [9]. In a large series of ultrasound-guided PCN by urologists, complications included urinary tract infection (1.1 %), hemorrhage (1.9 %), sepsis (0.76 %), inferior vena cava injury (0.15 %), gall bladder injury (0.15 %), and death (0.3 %). Overall, major complications occurred in 3.3 % of patients (3–6.7 % in various series) and minor complications in 5 % (5–38 % in various series) [10–12]. Successful PCN has been reported in these series as 90–95 %. PCN and PCNL under ultrasound guidance are safe and highly successful techniques in the hands of a trained urologist.
Percutaneous Renal Biopsy
Renal biopsy can be divided in two groups of patients: (1) patients with an abnormal mass to rule out malignancy or (2) patients with medical renal disease that requires histologic diagnosis. In both groups of patients, ultrasound imaging aids significantly to identify the suspected area. As discussed in other chapters of this book, the left renal US is often more challenging than the contralateral side due to the anatomic position.
Ultrasound probe: The use of curved-array 3.5–5-MHz probes with or without color Doppler provides good visualization and allows attachments that can display the path of the needle placement towards the target.
Technique: The technique is similar to PCNL . The target is different: (a) solid mass to rule out malignancy or (b) renal cortex to evaluate medical renal disease. As a general rule, percutaneous renal biopsy can be easily performed in nonobese patients and also transplanted kidneys since they are placed in the iliac fossa. The technique is similar to percutaneous renal procedures. If the colon is causing visual obstruction, rotate the patient and scan the kidney from posterior to anterior until the target is visualized. Preparation of the patient by fasting the night prior to the procedure may further improve visualization.
Clinical data: In the analysis of 623 renal biopsies guided by real-time ultrasound, the effectiveness was 97.6 % with 110 complications. Fourteen (2.24 %) were major complications (Clavien ≥3): nine cases of renal hematoma, two cases with macroscopic hematuria (which required blood transfusion), one case of intestinal perforation (which required exploratory laparotomy), one nephrectomy, and one case of a dissecting hematoma. The author concluded that the risk factors for developing major complications were the following: diastolic blood pressure ≥90 mmHg, RR 7.6 (95 % CI 1.35–43); platelet count ≤120 × 103/μL; RR 7.0 (95 % CI 1.9–26.2); and blood urea nitrogen (BUN) ≥60 mg/dL, RR 9.27 (95 % CI 2.8–30.7) [13].
Laparoscopic Ablative and Partial Nephrectomy
Historically, radical nephrectomy has been the treatment of choice for patients with renal masses, but our understanding and appreciation of nephron-sparing surgery for small renal masses ≤4 cm (and more recently, ≤7 cm in select patients) with regard to cancer control and preservation of renal function has increased dramatically in recent years [14–16]. There has been a recent migration towards nephron-sparing approaches as innovations with minimally invasive techniques continue [17]. The application of probe ablation therapies such as cryoablation and radio-frequency ablation, although promising, is currently not the treatment option of choice when compared to partial nephrectomy due to the lack of long-term outcome data. Although the percutaneous approach of the kidney for ablative procedures is feasible, the preferred imaging modality used to treat the renal masses by interventional radiologists has been CT scan. However amongst urologists, a laparoscopic approach using ultrasonography is more commonly implemented. Therefore, we will describe the use of laparoscopic ultrasonography for these procedures.
In the early 1990s, cryotherapy was reintroduced for the treatment of prostate cancer after study in animals and human trials in the 1970s and 1980s. On the basis of these experimental and clinical studies, an optimal drop in temperature of −40 °C is required to achieve total cell death [18]. Although many theories were suggested for the underlying mechanism of the cryoablative tissue effect, the vascular component of initial vasoconstriction followed by reperfusion injury (increased capillary permeability) triggered by the thawing phase is considered to be the primary mechanism of tissue damage. However, intracellular crystallization and subsequent water shifting during the thawing phase also contribute significantly to the rupture of the cell membrane and irreversible cellular death [19].
Ultrasound probe: Laparoscopic renal ultrasound will often be performed with a 6–10-MHz linear array transducer. Endoluminal probes of 10–12 MHz may be used for transurethral evaluations. The 7.5-MHz flexible side-viewing laparoscopic transducer offers distinct capability to contour the organ. The rigid 7.5-MHz linear side-viewing laparoscopic transducer is easier to operate and is preferred by surgeons. Both types of transducers use a 10-mm laparoscopic port for introduction to the abdominal cavity (Fig. 15.5).
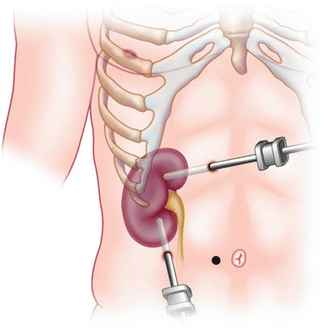

Fig. 15.5
Laparoscopic ultrasonic probe placement through a 10-mm trocar
Technique: LUS imaging and orientation may not be intuitive and easy to understand the exact position of the lesion or particular regions of the target. The universal orientation left/cephalad also applies to LUS, but prior to the scan one should tip the end of the LUS to visualize the left side of the monitor and run along the crystals of the probe to translate the images of the US with the location in the monitor. For example, if only the tip of the LUS probe is touching the lesion, the image will be displayed only on the right side of the monitor. As the probe travels from the tip to the base of the probe, the image will be displayed in the entirety of the monitor (Fig. 15.6).
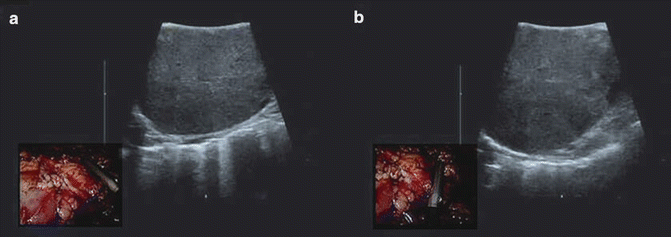

Fig. 15.6
Picture-in-picture of laparoscopic US during partial nephrectomy evaluates tumor size and depth to ascertain safe surgical margins. (a) lateral aspect of the renal tumor (b) medial aspect of the renal tumor
Indications for ultrasound-guided ablative therapy include small tumors (≤4 cm in diameter), older age, higher-risk patient, solitary kidney, and surgically scarred abdomen. Larger tumors and proximity to hilar vessels may be relative contraindications to ablative kidney surgery.
The size and optimal port placement are pivotal for LUS evaluation of renal lesions. The port size must be at least 10 mm. Generally, the posterior lesions are more challenging to be scanned. Either right or left side upper pole posterior lesions are best evaluated through an ipsilateral midclavicular or subxiphoid port. Anterior lesions can be evaluated almost through any port position. For a renal lesion biopsy and ablation, the needle or ablation probes are passed percutaneously through the abdominal wall. In general, multiple core biopsies (between 3 and 5) are taken with a 14- or 18-gauge needle under visual and ultrasonic guidance. Equally, the “rocking” maneuver allows the identification of the hyperechoic needle/probes and visualization of ablated tissue (Fig. 15.7).
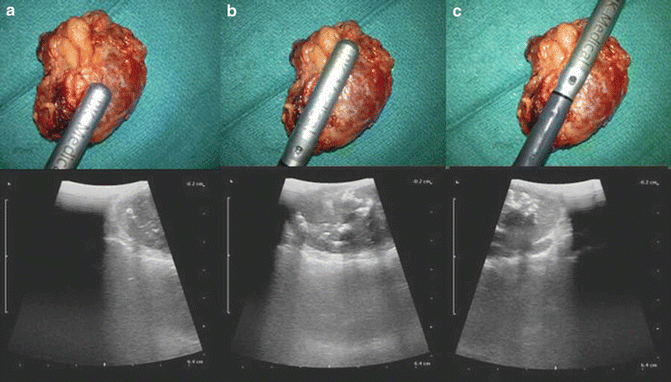

Fig. 15.7
Using the skiing technique across renal mass. The universal orientation of the laparoscopic probe is as follows: tip of the transducer is left and cephalad; therefore in (a) the distal end of the probe will demonstrate the adrenal mass on the right side of the monitor. When the transducer allows larger surface area contact, the image will show in the monitor (b) until only the base of the transducer is touching the adrenal mass and displaying the image on the left corner of the monitor (c) proximal end will demonstrate the adrenal mass on the left side of the monitor
Clinical data: A cryosystem consisting of argon (freezing phase) and helium (thawing phase) gases (Joule–Thomson effect) was used. Ultrasonic evaluation of the kidney should reveal suspect lesions. Simple cysts are generally spherical or ovoid, lack internal echoes, have a smooth, thin wall, and should show enhancement of the posterior wall indicating the presence of water. Lesions of suspect nature can include those with multiple septae, calcifications, or heterogeneous appearance that differentiates the lesion from healthy renal parenchyma [20]. The cryoneedles are introduced under real-time ultrasound guidance and will appear as hyperechoic structures with resultant shadowing. The borders of the ice ball can be readily identified on ultrasound as a hyperechoic rim (Fig. 15.8) which is generated by the interface between frozen and unfrozen tissues. The ice ball should be extended 1 cm beyond the visible border of treated lesions circumferentially to ensure negative margins. When thawed, the lesion will continue to remain hyperechoic compared with the surrounding kidney parenchyma [21]. All lesions should undergo two cycles of freezing and thawing.
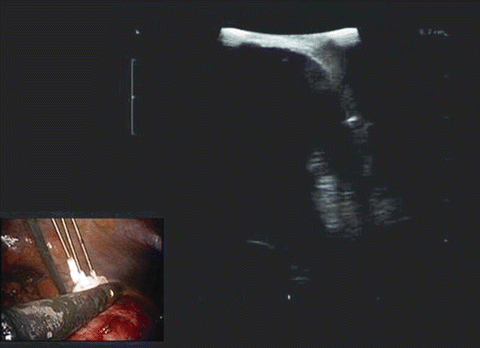

Fig. 15.8
Hyperechoic rim represents the outer layer of the ice ball. The homogeneous hypoechoic image is the ice ball that can be appreciated as the probes move towards the normal renal parenchyma
Robotic Partial Nephrectomy
Recently, ultrasound probes have been developed for robotic procedures including partial nephrectomies. Rogers et al. described the use of intraoperative ultrasonography for tumor identification during robotic partial nephrectomies (RPN ) [22]. A major drawback of laparoscopic ultrasonography during RPN is that the surgeon may rely on the assistant’s ultrasound skills, limiting the surgeons’ autonomy and precision. Due to these limitations, a robotic ultrasound probe controlled directly by the surgeon was developed [23]. The ProArt, a robotic transducer with doppler capability, was created by BK Medical in Herlev, Denmark [24]. It has a unique fin over the array which can be grasped by robotic instruments, giving the surgeon total autonomy over the probe. It can be maneuvered independently by the surgeon, achieving difficult angles while maintaining perpendicular contact of the probe with the kidney surface. Similarly, another fully articulated transducer by Hitachi-Aloka has been developed to achieve complex angles [24]. The DaVinci surgeon console has a software imaging program (Tilepro) that allows up to two adjunctive imaging inputs to be viewed by the surgeon simultaneously with the regular 3D camera field view. It has been recently demonstrated that robotic ultrasound probes for tumor identification during RPN had comparable perioperative outcomes and surgical margin rates to a laparoscopic ultrasound probe [25].
The Adrenal Gland
Ultrasonography of the adrenal gland is technically difficult and is better evaluated by other imaging studies, i.e., CT scan and MRI [26]. LUS is a valuable adjunct to laparoscopic adrenalectomy facilitating tumor localization and identification of adjacent structures to direct the dissection particularly for partial adrenalectomies. Although large lesions on either side are easily identified, laparoscopic US may facilitate identification of small tumors in obese patients and right-sided lesions that are usually partially retrocaval.
Ultrasound probe: Although the flexible side-viewing 7.5-MHz rigid laparoscopic transducer offers distinct capability to contour the organ, often surgeons find the rigid, linear side-viewing transducer the simplest and most convenient to use in adrenal surgery.
Technique: In the transabdominal technique , the patient is placed in the modified flank position with 3–4 trocars inserted subcostally on the right and three trocars on the left, on an axis between the midclavicular and the midaxillary lines. After the dissection along the gutter to deviate the liver or the spleen medially to expose the suprarenal retroperitoneum, the laparoscopic transducer is inserted through the 10- or 12-mm port on the anterior axillary line or subxiphoid port. If the adrenal gland has not been identified, the upper pole of the kidney should be scanned demonstrating characteristic appearance of its parenchyma with the cortex and the collecting system. Then the transducer is advanced cephalad in the longitudinal plane until the adrenal gland is identified. Large tumors are easily identified and the value of intraoperative sonography in these cases is in elucidating their relationship with the kidney, aorta, spleen, and the tail of the pancreas on the left and the kidney, inferior vena cava, and the liver on the right side, respectively. The use of flow color Doppler also helps to identify the aorta and the inferior vena cava. Nonsecreting and secreting adenomas have a similar ultrasonographic appearance. They are usually less than 3 cm and have a very low echodensity. They sometimes contain cystic and calcified areas. Pheochromocytomas may appear as solid or cystic or may have both solid and cystic components. Hypoechoic areas represent necrosis and hyperechoic areas indicate hemorrhage. The ultrasound appearance of adrenal hyperplasia is of smooth enlargement with a normal echo pattern. Adrenal cortical carcinoma of 3–6 cm shows a homogeneous echo pattern similar to renal cortical tissue. Larger lesions vary in ultrasonographic appearance, having a heterogeneous appearance with focal or scattered hypoechoic or hyperechoic zones representing areas of tumor necrosis, hemorrhage, or, rarely, calcification. Metastatic adrenal tumors usually have an ovoid shape and variable echogenicity (Fig. 15.9). Adrenal cysts appear as anechoic masses with enhanced posterior sound transmission, whereas myelolipomas are highly echogenic because of their fat content. Although the use of LUS facilitates tumor evaluation and identification of adjacent structures, especially large vessels, in cases of pheochromocytoma and adrenal cortical carcinomas, compression of the adrenal gland may have very harmful effects either triggering catecholamine release or rupture of the malignant tumor causing spread of cancer.
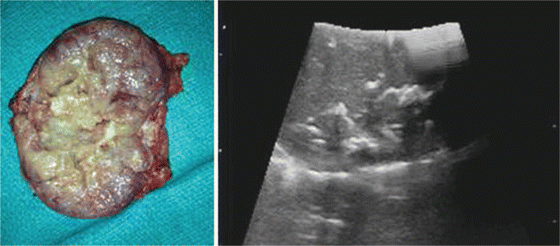

Fig. 15.9
An adrenal gland with ovarian metastasis. Gross anatomy with internal calcifications identified during laparoscopic adrenalectomy and use of LUS
Clinical experience: Contrast-enhanced ultrasonography has been employed in Europe and other countries to evaluate hypervascularity and correlate with malignancy. Hijioka et al. reported their experience on contrast-enhanced endoscopic ultrasonography (CE-EUS) to identify adrenal mass hypervascular lesions and perform EUS-guided fine needle aspiration (EUS-FNA). They concluded that this imaging modality can assist in the diagnosis of metastatic lesions, i.e., clear cell carcinoma leading to adrenalectomy and confirmation of metastatic clear cell carcinoma of the kidney [27]. Additional reports of ultrasound and/or CT scan-guided percutaneous cryoablation of adrenal masses have shown encouraging cost-effective outcomes. This procedure was associated with very low morbidity and local tumor recurrence rates and increased overall survival. Even as an adjunct to systemic therapies, it seems that percutaneous cryoablation of adrenal masses appeared cost-effective for palliation [28].
The Bladder
Suprapubic Tube Placement or Suprapubic Aspiration
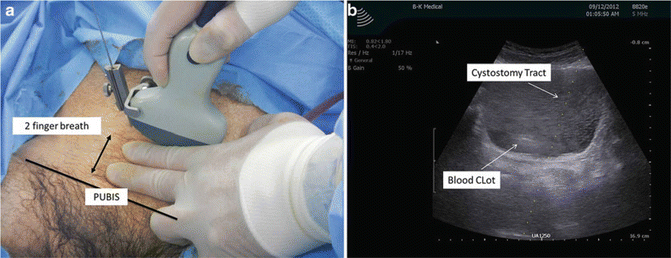
Aspiration or trocar suprapubic tube placement (SPT ) or “punch” suprapubic tube, as they are commonly referred, can offer quick drainage of the urinary bladder. The clinical scenario that necessitates SPT placement is the inability of the patient to void (posterior urethral disruption, urethral stricture) or when invasive urethral procedure may trigger sepsis (acute prostatitis) or autonomic dysreflexia (spinal cord injury patients) [29]. The primary risk associated with this technique is perforation of bowel overlying the urinary bladder and open SPT approach must be applied in these patients.
Ultrasound probe: The use of curved-array 3.5–5-MHz probes with or without color Doppler provides good visualization and allows attachments that can display the path of the introducing needle towards the bladder. In the absence of this type of probe , a linear array can be used to rule out bowel interposition between the skin and the bladder.
Technique : For ultrasound-guided trocar (SPT) placement or aspiration, the urologist should inquire the past surgical history of the patient, as prior abdominal operations could signify increased risk for bowel overlying the urinary bladder. On exam, the patient should have a distended bladder prior to the procedure and no scars that include the lower abdomen. Typical ultrasonography findings should include a variable-thickness bladder wall and dark homogeneous fluid content representing urine. One may survey the infraumbilical area and detect bowel contents represented by dark dynamic shadowing images representing peristalsis with gas content or any intervening tissue planes or heterogeneous echogenicity between the abdominal wall and urinary bladder. Presence of large blood clots also interferes with the hypoechoic characteristic of a full bladder. Further ruptured bladders will be difficult visualizing since the bladder may be empty. “Rocking” of the US probe will verify needle and catheter placement in a full bladder, and immediate urine drainage should be observed after initial puncture. The US probe follows the universal left/cephalad position and it should be positioned approximately three fingerbreadths above the pubic bone allowing the SPT needle to be placed between the US probe and the pubic bone (Fig. 15.10).