Stable medical condition amenable to surgery
*
Clear organic pain generator
*
No psychological or sociological contraindication
*
No familial contraindication such as severe codependent behavior
*
Documented responsible behavior and stable social situation
*
Good pain relief with oral or parenteral opioids
*
Intolerable side effects from systemic opioid therapy
*
Baseline neurological exam and psychological evaluation
*
Failure of more conservative therapy including trials with non-opioid medications and nerve blocks
*
Constant or almost constant pain requiring around-the-clock opioid therapy
*
No tumor encroachment of thecal sac in cancer patients
*
Life expectancy >3 months
*
No practical issues that might interfere with device placement, maintenance, or assessment (e.g., morbid obesity, severe cognitive impairment)
*
Positive response to an intrathecal trial
*
Proper patient evaluation also requires knowledge of the contraindications of intrathecal therapy (see Table 42.2) [10, 11].
Table 42.2
Contraindications for intrathecal (IT) therapy
Systemic infection |
Coagulopathy |
Allergy to medication being used |
Inappropriate drug habituation (untreated) |
Failure to obtain pain relief in a screening trial |
Unusual observed behavior during screening trial |
Poor personal hygiene |
Prior to implant, it is important to perform thorough patient counseling on intrathecal therapy to ensure an appropriate chance of success. The counseling process begins with detailed instruction on the entire procedural process, the risks and benefits of the procedure, and discussion on realistic expectations from the therapy. The patient must understand the signs and symptoms of potential under- and overdosages to minimize potential life-threatening problems (Table 42.3). The patient must also be instructed to avoid any strenuous and high-impact activities. Scuba diving at a depth of 2 ATA (atmospheres absolute) may damage the pump. These activities may potentially damage the intrathecal catheter or pump reservoir. Intrathecal catheter or battery malfunction may require revision secondary to catheter kinking or occlusion.
Table 42.3
Drug concentrations and dosages
Drug | Maximum concentration | Maximum dose/day |
|---|---|---|
Morphine | 20 mg/mL | 15 mg |
Hydromorphone | 10 mg/mL | 4 mg |
Fentanyl | 2 mg/mL | No known upper limit |
Sufentanil | 50 μg/mL (not available for compounding) | No known upper limit |
Bupivacaine | 40 mg/mL | 30 mg |
Clonidine | 2 mg/mL | 1.0 mg |
Ziconotide | 100 μg/mL | 19.2 μg (Elan recommendations) |
There is no standard interpretation of the efficacy and utility of an intrathecal trial. In general, minimal expected outcome is a >50 % reduction in patient’s pain and absence of side effects. Trial techniques are based on individual physician preferences and available resources. There is no convincing evidence that one technique is far superior to another; these include a single-shot injection of intrathecal opioids and non-opioids, multiple bolus injections, and continuous infusion of analgesics, either single or in combination, via an external catheter. There are pros and cons to each method.
A single intrathecal opioid injection involves the injection of intrathecal morphine or other opioids through a lumbar puncture. This is performed with a recommended 0.2–1 mg of intrathecal morphine or the daily equivalent intrathecal dose [12]. There are currently no standard conversion guidelines from systemic opioid doses to intrathecal dosing. Intrathecal boluses allow for a short trial period to evaluate the efficacy and safety of test medication. Long-term efficacy is not expected, and potential side effects of the therapy may occur but are usually short-lived. Multiple bolus injections can be performed through intrathecal or epidural injections, with or without the use of a catheter. This method allows the patient to experience a longer trial period to judge the efficacy of the therapy. The patient may also receive placebo injections to rule out any false positives or any underlying central mediated pain syndromes. The last method of trialing involves the use of continuous infusion of opioid therapy through an external intrathecal catheter. Continuous infusion occurs over several days at a low starting dose and increases every 6–12 h until pain relief is achieved [12, 13]. It is recommended that morphine be trialed in an inpatient or overnight setting to closely monitor for delayed onset adverse events, such as respiratory depression.
Non-opioids, such as ziconotide, may be trialed on an outpatient basis. Trialing of ziconotide is more complex and unpredictable mainly because of a narrow therapeutic window. Continuous infusion trials of ziconotide are traditionally utilized; however, some clinicians perform single bolus trials with varying dosages. Serious adverse side effects during a ziconotide trial, such as respiratory depression and death, are unlikely [13]. Although life-threatening adverse events are not expected with ziconotide, appropriate monitoring for cognitive and psychological effects, ataxia, nausea, and vomiting is recommended.
Psychological Evaluation for Implantable Devices
Currently, there is no large prospective study demonstrating positive predictive value of preoperative psychological testing prior to implantation of IT pump. However, most experts agree that psychological evaluations can improve the patient’s chance of successful therapy by discovering untreated depression, anxiety, drug addiction, or/and underlying personality disorders. Patients with underlying personality disorders may have poor functional outcomes using implantable pain therapies [14]. Patients should understand possible outcomes and have realistic expectations for intrathecal therapy. A therapeutic partnership with the patient and physician should focus on adherence to the physician’s recommendations and self-monitoring of efficacy and adverse events.
Intrathecal Delivery Systems Implantation Technique
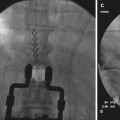
The first step in the implant process is to appropriately prepare the patient for surgery. This begins by marking the potential pump reservoir site on the patient’s abdomen. This should be performed with the help of the patient making sure that the site will not interfere with wheelchair use, patient’s beltline, or any other activities of daily living. The most appropriate position in the abdomen would be below the lower costal margin and above the iliac crest and beltline and away from rectus abdominis muscle [15]. Chlorhexidine wash one night prior to implantation is recommended by some clinicians; however, there is no clear evidence that this reduces surgical site infection. After appropriate preoperative IV antibiotics are given, the patient is taken to the operating table and placed in the lateral decubitus position with the pump site in the nondependent position. Sterile prep and drape is then performed. A spinal needle is then placed at a shallow-angle (approximately 30° off the spine), paramedian oblique needle insertion trajectory (see Fig. 42.1). The entry point of the needle into the skin (or fascia if the needle insertion is performed through an open incision) should be approximately 1–1 1/2 vertebral levels below the interlaminar space selected for dural puncture and 1–2 cm lateral to the midline, on the side of the intended pump pocket.
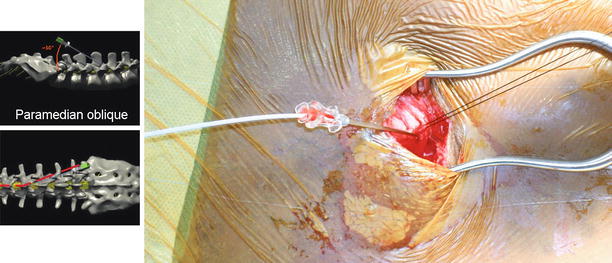

Fig. 42.1
Lateral and anterior-posterior model view of intrathecal needle placement using paramedian oblique approach. Note a significantly decreased angle to the lumbar spine. Such acute angle is needed to easily position catheter into posterior intrathecal space (Modified from the Medtronic IT implantation manual)
The catheter guide wire is seated completely, with its hub against the proximal end of the catheter and remains in place during all maneuvers to insert or position the catheter. The needle bevel is oriented cephalad and the distal tip of the catheter is threaded through the needle to the desired location. One must be aware that if the catheter must be retracted during positioning, the needle tip can damage the catheter, requiring additional surgery to repair or replace the catheter. The needle is carefully removed, ensuring that the hub is orientated cephalad, with the guide wire removed simultaneously. To prevent catheter damage or dislodgement during guide wire removal, the catheter is held straight and securely at the exit site. Minimal traction to avoid catheter twisting helps prevent damage to the catheter. A purse string suture is then tied around the fascia surrounding the catheter, and then, an anchor is attached to the fascia via nonabsorbable sutures.
A subcutaneous pocket is prepared that is large enough to accommodate the selected IT pump. The pocket size should be close-fitting to prevent flipping or migration of pump. Expert implanters recommend that the pocket size should be no more than 20 % larger than the size of the pump. Using the appropriate size catheter passer, a subcutaneous tunnel is formed from the spinal incision site directed toward the pump pocket. The residual catheter length should be noted for accurate programming if a programmable pump is implanted. Strain relief loops at the spinal incision site and behind the implanted pump will allow for patient movements.
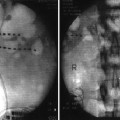
Nonabsorbable sutures are placed in the pump pocket fascia closest to four different corners of the pump pocket. The pump is then positioned inside the pocket so that the catheter is not twisted or kinked and securely anchored (Fig. 42.2). Skin and underlying fascia are closed with sutures.
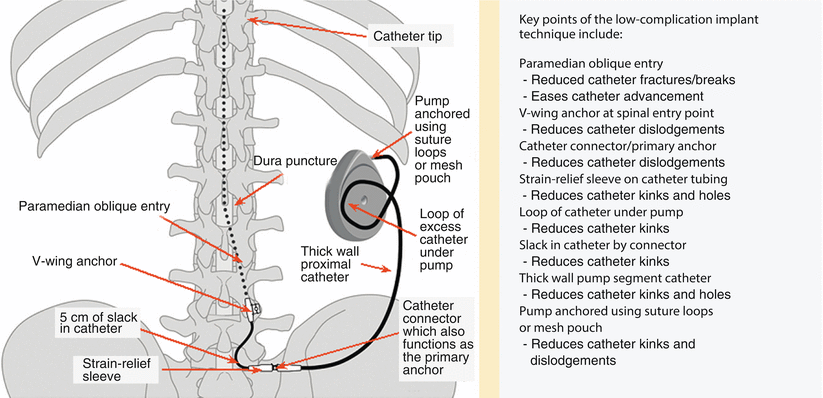

Fig. 42.2
Schematic and key points of what is called “low-complication implant technique” for intrathecal pump and catheter. Although such recommendations seem to represent common sense, there are no studies to support all of listed recommendations to prevent catheter kinks and migrations (Modified from the Medtronic IT implantation manual)
Basics Concepts of Intraspinal Drug Delivery Routes
Epidural versus intrathecal modes of delivery influences distribution of the delivered drugs. Drugs delivered epidurally must cross the dura and arachnoid and then diffuse to their site of action. Drugs delivered intrathecally diffuse directly into the spinal cord [18]. However, the location of the catheter is important even for intrathecal drug delivery. Adjustment of intrathecal medications may be required to achieve therapeutic concentrations at target sites in the spinal cord if the catheter is distal to the targeted spinal level [16, 17].
The hydrophilic medications circulate throughout the CSF. Their duration of action is longer, have relatively slow onset, rostral migration may be more predictable, and is dose dependent [18]. Drugs deposited intrathecally reach high CSF concentration rapidly before reaching any significant serum level and are dose dependent [19]. Morphine and other opioids diffuse to the substantia gelatinosa in the spinal cord – their primary site of action – and bind to opioid receptors there. The required dose to achieve pain relief epidurally is much higher than intrathecally, and systemic absorption is much higher for the same level of analgesia. There is approximately five to ten times reduction of required dose when the route of morphine delivery is changed from epidural to intrathecal [20].
Lipophilic medications remain closer to the catheter tip. For these medications, slow migration of the drug in the spinal space, combined with the rapid uptake by intrathecal tissues, produces large drug gradients within the intrathecal space. Lipophilic opioids (fentanyl and sufentanil) when used for epidural analgesia have significantly higher plasma levels and exhibit less rostral spread. Their spinal mechanism of action is uncertain [21]. The dose for epidural injection is higher than for intrathecal administration, and drug pharmacokinetics is more complex. Dural penetration, epidural fat deposition, and systemic absorption occur [21]. The extent of systemic absorption for epidural infusion is thus higher than with intrathecal administration.
Recent animal studies provided the evidence that the position of the infusion catheter orifice and its relationship to the targeted spinal cord segment are crucial. There is limited capacity of the CSF to distribute drugs away from the distal lumen of the catheter [22–24]. Inadequate pain relief following definitive pump implantation occurs frequently and often requires optimization, even after a successful trial.
The infusion velocity of the drug and the CSF motion are two elements that are critical in intrathecal pharmacokinetics. A bolus or a faster infusion rate shows wider CSF distribution compared with slower rates. The classic flow of CSF is caudad in the posterior surface of the spinal cord and cephalad along the anterior surface. However, this classic depiction is incorrect based on multiple MRI studies that have showed a to-and-fro rostrocaudal movement, driven by the cerebrospinal vasculature during the cardiac cycle. This flow pattern is greatest in the upper cervical segments and decreases progressively, becoming negligible at the cauda equina. There are three channels of CSF flow. These are medial-ventral, medial-dorsal and lateral, and the most valuable is the undetected circumferential motion in between the anterior and posterior sides of the spinal cord. The dorsal horn is the target. A well-positioned posterior catheter tip is important for dorsal column flow; however, targeting the anterior horn via ventral placement of the catheter tip may provide a better drug response [24].
Intrathecal Versus Epidural Delivery Route
When compared to epidurally placed catheters, intrathecal catheters may last longer with lower rates of complications [25, 26]. In addition, side effects related to drug infusion are less frequent (lethargy, respiratory depression, dysphoria) when intrathecal route is used [27]. The development of tolerance in patients with chronic pain is less frequent when intrathecal route is used [28]. Moreover, the major reason to select the intrathecal route for delivery of opioids came from cancer pain research. Patients who converted from epidural to intrathecal morphine have somewhat better pain relief [25, 26]. This trend was most obvious when long-term externalized epidural catheters were followed by externalized and internalized intrathecal catheters [26]. In regard to medications used, epidural opioids either alone or combined with epidural bupivacaine provided significantly less pain relief than intrathecal opioids or intrathecal opioids and bupivacaine [26].
Intrathecally Delivered Medications
Morphine
Morphine is the prototypical opioid analgesic. It is the only opioid currently approved for intrathecal administration by the FDA. Morphine is a highly hydrophilic compound that is poorly metabolized in the CSF, leading to prolonged duration of effect when delivered intrathecally. Morphine works through activation of opioid receptors, most specifically the μ-opioid receptor. These receptors are located in both the superficial laminae of the spinal cord and supraspinal sites such as the periaqueductal gray matter and the raphe nuclei [29]. Mu opioid receptors are G protein-coupled receptors and are located at both pre- and postsynaptic sites in the spinal cord. On presynaptic terminals, activation of μ-opioid receptors leads to inhibition of voltage-gated calcium channels [30, 31]. In primary afferent neurons, this results in decreased substance P and excitatory amino acid release [32, 33]. Agonism of postsynaptic μ-opioid receptors activates G protein-coupled inwardly rectifying potassium (GIRK) channels leading to neuronal hyperpolarization [34, 35].
Hydromorphone
Hydromorphone is a semisynthetic derivative of morphine. Like morphine, it is a μ-opioid receptor agonist, but it also activates δ- and κ-opioid receptors. Hydromorphone is commonly used as a first-line drug for intrathecal delivery. In addition, due to its different chemical properties and receptor pharmacology, it is also used as a second-line agent when morphine fails to provide sufficient analgesia [36]. There is some evidence that hydromorphone is more lipophilic than morphine which may account for decreased distribution to supraspinal sites resulting in less side effects [37]. The potency of hydromorphone is five times that of morphine.
Fentanyl and Sufentanil
Fentanyl and sufentanil are synthetic anilinopiperidines that are μ-opioid receptor agonists. They are both highly lipophilic which results in segmental analgesia near the catheter tip due to rapid diffusion out of the CSF into the systemic circulation. Anilinopiperidines have higher intrinsic receptor activity than morphine, indicating that less receptors need to be occupied to generate the same physiological response [38, 39]. This property is likely related to the decreased tolerance seen with anilinopiperidine opioids compared to morphine. Relative to morphine, the potencies of fentanyl and sufentanil are 100 and 1,000 times greater, respectively.
Clinical Evidence for Intrathecal Opioids
Intrathecal opioids are currently used in the treatment of a broad spectrum of clinical conditions. Despite an extensive clinical literature detailing the usage of intrathecal opioids, only a relative handful of controlled prospective studies exist on the topic. The strongest evidence for the use of intrathecal opioid comes from the treatment of cancer pain. Rauck and colleagues published a multicenter prospective study of 119 cancer pain patients with data reported out to 16 months. Intrathecal morphine reduced the VAS from 6.1 to 4.2, and this effect continued through month 13. In addition, the patients had a reduction in both oral opioid consumption and the opioid complication severity index. The authors reported clinical successes of 83 % at 1 month and 91 % at 4 months with success defined as ≥50 % reduction in VAS, use of systemic opioids, or opioid complication severity [40]. Smith and colleagues published an important prospective multicenter randomized clinical trial comparing comprehensive medical management (CMM) versus CMM plus implantable drug delivery system (IDDS). With clinical success defined as ≥20 % reduction in VAS or equal VAS with ≥20 % reduction in toxicities, the CMM plus IDDS success rate was 84.5 versus 70.8 % for CMM alone. At 4 weeks, the CMM plus IDDS VAS was reduced by 52 versus 39 % for the CMM alone group. In addition, the CMM plus IDDS group had significant reductions in fatigue and depressed levels of consciousness. Finally, the CMM plus IDDS group had a trend toward increased survival at 6 months [7]. Collectively, these studies indicate a strong role for intrathecal opioids in managing cancer pain.
Evidence for the use of intrathecal opiates in noncancer pain is not quite as clear as with cancer pain in part because of a lack of randomized controlled trials. That said, several high-quality prospective studies have evaluated the effect of intrathecal opioids on neuropathic, nociceptive, and mixed noncancer pains [41–47]. Deer and colleagues reported a multicenter prospective registry of 136 patients with low back and leg pain who were implanted with intrathecal drug delivery systems. At 1 year, numeric pain ratings dropped by 47 % for back pain and 31 % for leg pain. In addition, there was a significant improvement in the Oswestry Low Back Pain Disability scores in implanted patients. Patients who had successful trials but were not implanted did not have similar improvements [48]. Thimineur and colleagues reported a prospective three-armed study of chronic nonmalignant pain. The first arm (N = 38) were implanted with pumps, the second (N = 31) either failed a trial or declined implantation, and the third (N = 41) were newly referred patients. All participants filled out extensive questionnaires at baseline and every 6 months for 3 years. Intrathecal therapy had significant benefits on pain scores, functionality, and mood. The nonimplanted group declined in these functions despite escalation of oral opioids and injective therapies. Neither of the first two groups advanced as well as the new referral group indicating that although intrathecal therapy is associated with significant pain, functional, and mood improvements, the disease pain burden remains high [49]. Although ample evidence exists demonstrating the ability of intrathecal morphine to provide analgesia for chronic noncancer pain, randomized trials need to be conducted before it is considered standard care.
Non-opioids
Bupivacaine
Bupivacaine is the most commonly used local anesthetic for intrathecal administration. The drug is an amino amide that binds the intracellular portion of the α-subunit of voltage-gated Na+ channels, inhibiting Na+ influx into the neuron. This ultimately decreases the rate of neuronal depolarization, inhibiting action potential initiation and signal transduction [50]. For continuous intrathecal infusion therapy, bupivacaine is commonly combined with other agents and is rarely used alone. The drug is generally selected for neuropathic pain or when analgesic doses of opioids produce intolerable side effects. The data regarding the efficacy of intrathecal bupivacaine are mixed. In a retrospective analysis of 109 patients with failed back surgery syndrome or metastatic cancer, Deer and colleagues reported that patients with intrathecal opioids plus bupivacaine had better pain control, fewer physician visits, and higher overall satisfaction than patients with intrathecal opioids alone [51]. A single randomized, double-blind placebo-controlled crossover study has evaluated the effect of adding bupivacaine to intrathecal opioid therapy. Mironer and colleagues utilized 24 patients with chronic nonmalignant intractable pain and found no difference in mean pain scores with the addition of bupivacaine; however, there was a statistically significant improvement in quality of life scores [52]. Additionally, one prospective study in cancer patients by van Dongen and colleagues demonstrated that the addition of bupivacaine to morphine attenuated the dose progression of morphine over time, indicating a likely synergistic effect between the two drugs [53].
Ziconotide
Ziconotide is a selective N-type calcium channel blocker that was formerly known as SNX-111. It is a synthetic analog of the ω-conopeptide derived from the venom of the giant marine snail Conus magus [54, 55]. Ziconotide inhibits presynaptic N-type calcium channels, leading to decreased excitatory neurotransmitter release in the dorsal horn. Ziconotide is a permanently charged molecule with a molecular weight ten times that of morphine which provides relative confinement to the CSF. In addition, the molecule is relatively resistant to CSF peptidases. As such, clearance of the drug is mediated by CSF bulk flow and not by metabolism [56]. Common side effects include amblyopia, dizziness, nausea, nystagmus, urinary retention, and vomiting, which appear to be in part dose and titration schedule dependent. Ziconotide is approved for intrathecal administration by the FDA.
Three double-blind placebo-controlled studies examining the efficacy of ziconotide in the treatment chronic pain have been conducted. Staats and colleagues evaluated 111 patients with cancer or AIDS-related pain over an 11-day span. The study included a 5–6 day titration phase followed by a 5-day maintenance phase. The placebo group was crossed over into the ziconotide group after 5 days. Mean VAS reduction at the end of the titration phase was 53 % for ziconotide versus 18 % for the placebo group [57]. Wallace and colleagues evaluated 169 with nonmalignant mostly neuropathic pain over 11 days. Mean VAS reduction was 31 % for the ziconotide group versus 6 % for the placebo-treated group. Although both of these studies had significant reductions in pain scores with ziconotide therapy, they also had higher rates of serious adverse events and discontinuation [58]. Rauck and colleagues enrolled 248 patients with neuropathic, nociceptive, and mixed pain for randomization into either intrathecal ziconotide or placebo treatment. Unlike the previous two studies, the dose of ziconotide was gradually increased over 3 weeks to a low maximum dose. At week 3, the VAS decreased by 14.7 % in the ziconotide group compared to 7.2 % in the placebo group (P = 0.036). Discontinuation rates for adverse events and serious adverse events were similar between the groups [59]. Collectively, these results demonstrate that intrathecal ziconotide is an effective analgesic for both cancer and noncancer pain and that the adverse effects of the drug can be limited by careful titration and dosing.
Clonidine
Clonidine is a relatively selective α-2-adrenergic receptor agonist that produces dose-dependent analgesia when delivered intrathecally. It is FDA approved for epidural use in the treatment of cancer pain [60, 61] but is commonly used for intrathecal therapy [62]. Alpha-2-adrenergic receptors are located on both pre- and postsynaptic neurons in the dorsal horn [63–65]. The mechanism of action is mediated through inhibition of substance P and excitatory amino acid release from the primary afferent neurons and direct inhibition of second-order neurons [29, 66]. Clonidine synergistically augments the effect of morphine. It does not cause respiratory depression and does not potentiate opioid induced respiratory depression [67]. The major side effects of clonidine are bradycardia and hypotension. These effects tend to be dose related [68]. Abrupt cessation of intrathecal clonidine therapy may produce severe rebound hypertension [62




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree