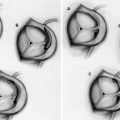
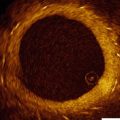
Fig. 8.1
Ultrasound waves are generated perpendicular to the IVUS catheter (Courtesy of Boston Scientific)
There are currently two different types of IVUS catheter designs; solid state and rotational and both types of systems generate a circumferential, gray scale axial image (See Fig. 8.2)
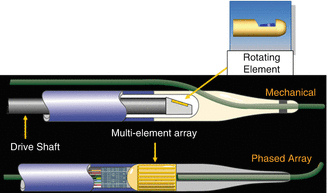

Fig. 8.2
Top image is a rotating, or mechanical catheter. Bottom image is a solid state, or phased array catheter (Courtesy of Boston Scientific)
1.
Solid–state (Phased Array) IVUS:
With solid-state catheters, there are multiple transducer elements that are mounted circumferentially at the tip of the imaging catheter. These are activated individually in a rotational fashion in order to generate a rotating ultrasound beam. The resulting echocardiographic information is then routed to a computer system, which in turn generates a cross sectional, real time image. The only commercially available solid-state catheter currently available (Volcano) has 64 separate transducer elements arranged around the catheter tip and uses a 20 MHz scanning frequency. These catheters are 3.5 French in size at the transducer and are compatible with a 5 French guiding catheter over a 0.014-in. guide-wire in a rapid exchange design. Larger devices are also available for use over both 0.018 and 0.035-in. wires and are designed for use in the peripheral vessels and aorta. Aside from conventional IVUS images, the Volcano solid-state catheters also perform radiofrequency IVUS, also known as virtual histology or VH-IVUS which will be discussed in a later section.
2
Rotational (Mechcanical) IVUS.
With rotational systems, a single transducer element is located at the tip of the catheter that is rotated by an external motor drive attached to the proximal end of the catheter. As the transducer rotates, echocardiographic information is gathered and generated into a circumferential cross sectional image, identical to that generated by solid-state systems. As the rotating transducer sits inside the catheter, there is a very short rapid exchange portion at the tip of the catheters for use with a 0.014 in. guide-wire. Currently, rotational coronary imaging systems are available in three separate platforms (Boston Scientific, Volcano and Infraredx). The Boston Scientific coronary catheter has a 3.15 French crossing profile, is compatible through a 5 French catheter, and uses a 40 MHz scanning frequency. The Volcano rotational catheter has a 3.2 French crossing profile and is compatible through a 6 French sheath and uses a scanning frequency of 45 MHz. The Infraredx device has a 3.2 French crossing profile, is compatible through a 6 French sheath and uses a 40 MHz scanning frequency. The main difference in the Infraredx catheter is that it also provides the ability to perform near infrared spectroscopy (NIRS) in addition to IVUS (See Fig. 8.3).
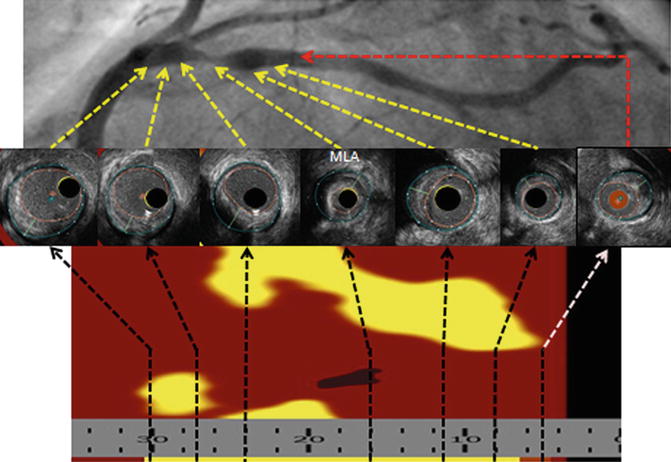

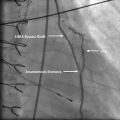
Fig. 8.3
InfraRedX IVUS images. The yellow in the underlying longitudinal image represents lipid, which can be quantified into a lipid core burden index (LCBI)
For coronary imaging, both solid state and rotational systems are performed over a 0.014 in. guide-wire which is placed by the operator across the area of interest and into the distal vessel after fully anticoagulating the patient. The majority of interventionalists will then give a single dose of 100–200 micrograms of nitroglycerin intracoronary in order to have maximal epicardial vasodilation as well as to minimize coronary spasm induced by the imaging catheter which is a complication in approximately 2 % [4]. The IVUS catheter is then advanced over the guide-wire with the transducer beyond the area of interest. After initiation of the IVUS catheter, baseline circumferential images are obtained.
There are two different methods of then proceeding with IVUS, which are manual pull back, or motorized pullback, which can be done with any of the currently available catheters.
In manual pull back, the operator will slowly withdraw the transducer across the area of interest. With solid-state catheters, the entire catheter is slowly pulled back while with rotational catheters the internal imaging catheter is slowly withdrawn leaving the outer catheter in place beyond the lesion. With motorized pull back, an external “sled” is attached to the proximal portion of the catheter which when activated will provide a steady withdrawal of the catheter at a standardized speed (See Fig. 8.4). The advantages of manual pullback are simplicity of operation and ability to “fine tune” the catheter to the area of interest for more detailed and prolonged investigations. The main advantage of motorized pullback is ability to provide information not only on vessel diameter, but also provide longitudinal information as the length of the area visualized is reported as well. This allows physicians to accurately determine lesion length in order to tailor not only the diameter of stents, but the length as well. With manual pullback one is not able to determine length or gather longitudinal information.


Fig. 8.4
Mechanical, or rotational, imaging system with overlying catheter. Note the single transducer which is rotated by an external drive shaft to create the cross-sectional image (Courtesy of Boston Scientific)
There are advantages and disadvantages of both the solid-state and rotational systems. With solid–state catheters setup is very simple: the catheter system is removed from its packaging, the proximal end plug is passed off and connected to the computer system, and the catheter is mounted on the 0.014 in. guide-wire over a rapid exchange segment followed by advancement to the area of interest. Because solid-state catheters have a zone of “ring down” artifact around the catheter, an additional step to mask this artifact is performed once the catheter is advanced into the coronary, which is done, on the computer console. Other advantages of the solid-state catheters include the lack of moving parts, lack of guide-wire artifact, and lack of “NURD” which is a type of artifact with rotational systems which will be further explained in upcoming sections. Historically, the solid-state catheters were smaller in size, however with further advances in technology the crossing profiles of both the solid-state and rotational systems are essentially identical. In addition, due to a longer monorail segment there is less “buckling” of the catheter across tight stenoses leading to improved crossibility. Finally, one of the major disadvantages of the solid-state systems is the use of 20 MHz frequencies which allow imaging of larger vessels as well as increased penetration, however has less axial resolution and are felt by many interventionalists to provide less clear images. However, predominantly due to the simplified set-up and use there is still a very strong role for these catheters.
With rotational catheters, axial resolution is significantly better (38–50 μm compared to about 150 μm with solid-state catheters) due to the use of 40–45 MHz frequencies. However, the set-up for rotational systems is more cumbersome. The catheter is removed from its packaging and its associated stop-cocks and lines are attached. The motor drive is then handed over and placed in sterile bagging as it is a re-usable part. The catheter is then connected to the motor drive followed by flushing of the catheter in both the distal and proximal positions in order to remove all air from between the inner and outer catheters. The catheter, which has a very short monorail at the distal tip, is then placed on a 0.014 in. guide-wire and advanced through the catheter beyond the area of interest. If manual pullback is to be performed, it is important to only pull back the inner catheter during imaging leaving the stationary outer sheath in place, as opposed to pulling back the entire system as a unit. As the guide-wire runs alongside the transducer (rather than through the catheter as with solid-state systems), an imaging artifact inherent to all rotational systems is “guidewire artifact.” This is important to recognize in order to avoid misinterpretation of images.
Regardless of the type of system used, the generated axial image is essentially the same and interpretation and measurement relies upon the ability to correctly identify the different portions of the blood vessel as well as identify diseased and healthy appearing vessels. The three basic histologic layers and composition of the blood vessels provide the basis for image interpretation and result in the classic trilaminar appearance of IVUS. These layers are the intima, media, and adventitia (See Fig. 8.5). The innermost layer, the intima, is in direct contact with the intraluminal space and is typically only one to two cell layers thick in healthy arteries. Therefore, in truly normal coronaries, as seen in young individuals, the intima will not be able to be seen by IVUS because of the limits of the axial resolution. However, due to age as well as due to remodeling and deposition of atherosclerotic plaque from CAD, the majority of adults evaluated in the cath lab have a much thicker intima allowing its visualization by IVUS which appears echogenic. The intima is separated from the media by the internal elastic membrane which is composed predominantly of homogenous layers of smooth muscle cells in the coronaries. As the smooth muscle cells do not reflect sound waves well and there is less elastin and collagen as compared to the intima and adventitia, the media appears as a thin echolucent strip surrounding the vessel and separating the adventitia from the intima. The media is separated from the adventitia by the external elastic membrane. It is composed of fibrous connective tissue with a high amount of elastin and collagen and therefore appears echogenic. The imaged vessel wall therefore has, almost invariably, a classic trilaminar appearance (bright-dark-bright) providing important land marks for measurement (See Figs. 8.6 and 8.7).
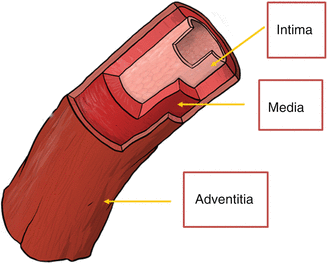
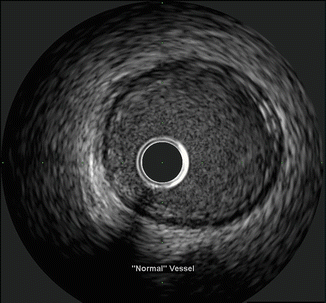

Fig. 8.5
Trilaminar structure of the blood vessels (Courtesy of Boston Scientific)

Fig. 8.6
Healthy vessel. Note the trilaminar appearance with a thin echogenic intima, echolucent media bounded by the internal and external elastic lamina, and echogenic adventitia (Courtesy of Boston Scientific)

Fig. 8.7
Cross-sectional and longitudinal images generated by IVUS. Note the calcified stenosis in the center of the longitudinal image (Courtesy of Boston Scientific)
As stated, the major strength of IVUS is its ability to see both the intraluminal as well as extra-luminal portions of the vascular bed in order to provide a more complete picture. It is also a very useful tool for the accurate and reproducible measurement of many parts of the blood vessel. This provides many obvious uses such as comparison of vessel diameter across a coronary stenosis and the reference vessel segment and the ability to accurately determine the size and length of coronary stents and balloons to be used during interventions. However, there are many other measurements which are commonly used in major clinical trials as cut points to guide interventions and therefore knowledge of how to determine these measurements is important. The following is a brief list of many of the commonly used measurements and terms which are thought be important for the practicing interventionalist. Later, discussions on clinical utility of many of these measurements will be covered. While determination of measures such as plaque burden and remodeling index are not routinely performed in every day clinical practice, the nomenclature is important as many of these have been used in previous studies.
Proximal Reference Vessel : The site with the largest lumen proximal to a stenosis but within the same segment, usually <10 mm from the stenosis with no intervening branches.
Distal Reference Vessel : The site with the largest lumen distal to a stenosis but within the same segment, usually <10 mm with no intervening branches.
Maximal Lumen Diameter: Maximal diameter of the lumen, from leading edge of intima on each side of vessel
Minimal Lumen Diameter : Minimal diameter of the lumen, from leading edge of intima on each side of vessel
Lumen Eccentricity :
Cross Sectional Area ( CSA ): Circumferential area bounded by the luminal border
Area Stenosis :
External Elastic Membrane Cross Sectional Area ( EEM CSA ): Circumferential area bounded by the leading edge of the external elastic membrane (EEM)
Atheroma or Plaque Area :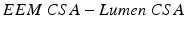
Atheroma or Plaque Burden: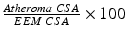
Remodeling Index ( RI ):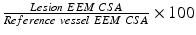
Artifacts and Pitfalls
Calcium
IVUS depends on differential absorption and reflection of sound waves from tissues in order to generate a grayscale image. Tissues that reflect large amounts of sound waves are termed echogenic and appear brighter relative to tissues that do not reflect sound waves as well, which are termed echolucent and appear darker. Calcium is an exceptional reflector of sound waves and therefore appears very echogenic. In addition, as the majority of sound waves are reflected and do not penetrate to the underlying tissue; calcium has significant shadowing making it difficult or impossible to see beyond the calcium, this is also knows as shadowing (See Figs. 8.8 and 8.9).
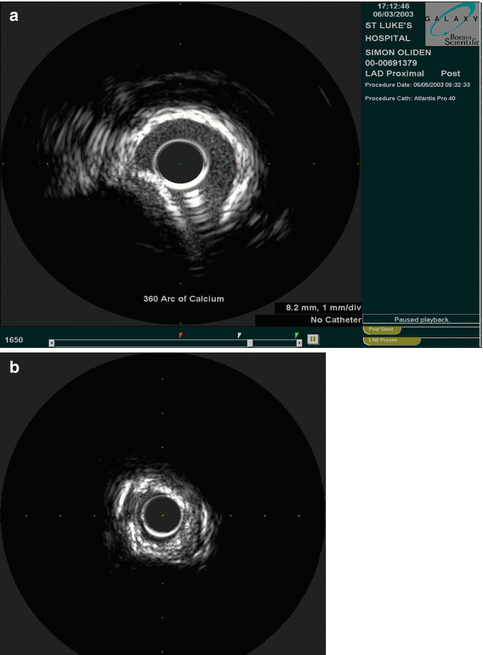
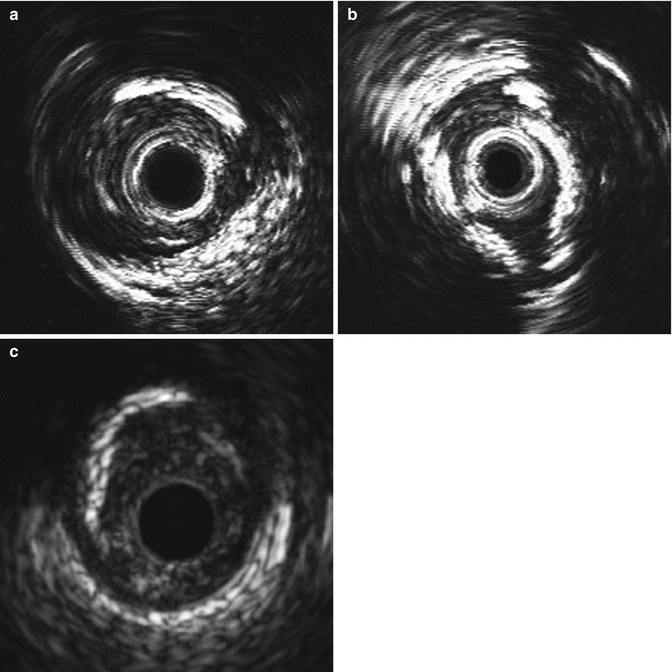

Fig. 8.8
(a) Calcified vessel with wire artifact in the 5 o’clock position (Courtesy of Boston Scientific) (b) Calcium artifact. Note the echogenic signal with acoustic shadowing beyond the calcium

Fig. 8.9
Calcium is described based upon its extent and location. (a) partially calcified vessel; (b) superficial calcium located closer to the lumen than the adventitia; © deep calcium located closer to the adventitia than the lumen (Courtesy of Boston Scientific)
Non-uniform Rotational Distortion (NURD)
This type of distortion is unique to rotational systems and results from mechanical binding of the drive cable that rotates the transducer. This is typically due to things which result in increased friction on the drive cable such as tortuous vessels or guide catheters, excessive tightening of the hemostatic valve on the guide, kinking of the imaging sheath, or too small a guide catheter lumen (See Fig. 8.10).
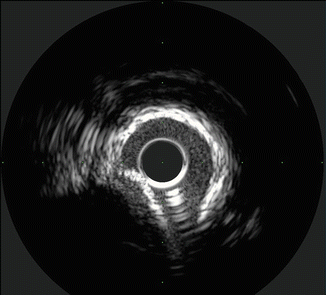

Fig. 8.10
Calcified vessel with wire artifact in the 5 o’clock position (Courtesy of Boston Scientific)
Wire Artifact
Similar to calcium, guide wires are very echogenic resulting in shadowing and difficulty seeing the vessel wall beyond the artifact. For those not familiar with this type of artifact misdiagnosis of thrombus or dissection is possible (See Fig. 8.11).
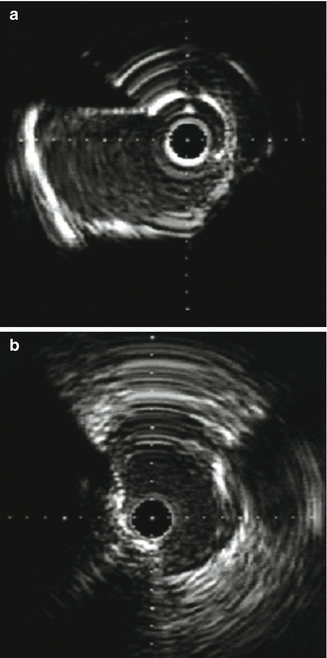

Fig. 8.11
(a and b) NURD artifact which is unique to rotational systems. Secondary to non-uniform rotation of the transducer due to increased friction on the catheter. Note the concentric ring artifacts that are generated (Courtesy of Boston Scientific)
Motion
Both mechanical and solid state catheters can move as much as 5 mm longitudinally between diastole and systole [5] which can preclude accurate assessment, especially with longitudinal measurements.
Ringdown
This type of artifact refers to disorganization of the image closet to the transducer or catheter. While present in all types of IVUS catheters it is more common in solid state, or phased array IVUS catheters which attempt to minimize these artifacts by performing a “ringdown” on placement of the catheter into the vessel, which is simply a mask or digital subtraction over this area in the center of the image. Ringdown artifacts are observed as bright halos of variable thickness surrounding the catheter. They are produced by acoustic oscillations in the transducer which result in high-amplitude signals that obscure the area immediately adjacent to the catheter [4].
Blood Speckle
Signals from blood cells can be imaged on IVUS catheters. This “blood speckle” artifact increases as transducer frequency increases and as blood flow decreases. Therefore areas where the catheter is across a tight stenosis with resultant limitations in blood flow, blood speckle artifact increases. This can result in decreased ability to differentiate lumen from tissue, especially with soft plaque and thrombus. Flushing the catheter with saline helps to clear the lumen and reduce blood speckle, which in turn may aid in identification of tissue borders (See Fig. 8.12).
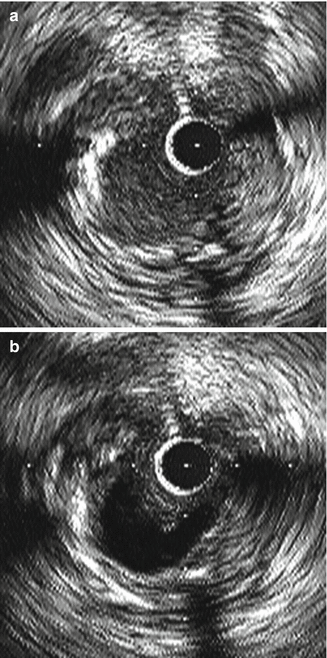

Fig. 8.12
Blood speckle artifact noted in the image on the (a) secondary to slow flow. This is improved in the image on the (b) after flushing of the catheter (Courtesy of Boston Scientific)
Patient Populations
Assessment of Intermediate Non-left Main Coronary Lesions
Because angiography is the planar 2D representation of a 3D structure, there are multiple potential pitfalls in accurate interpretation. Diffuse reference vessel disease, foreshortening, tortuous vessels, overlapped segments, calcification, lesion eccentricity, and poor contrast opacification all can contribute to inaccurate assessment of lesion severity. Because of the tomographic representation of the vessel as well as the ability to evaluate the vessel wall it is not surprising that IVUS can be very useful in the assessment of the “intermediate” or “ambiguous” coronary stenosis, defined as angiographic stenosis of 40–80 % in most trials, and in fact has been shown to be more accurate and reproducible than angiography and quantitative coronary angiography (See Fig. 8.13).
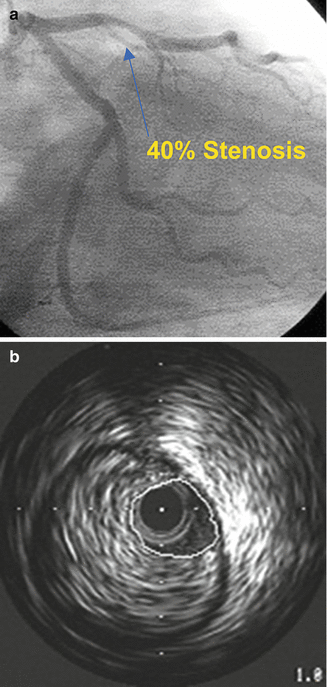

Fig. 8.13
Eccentric plaque which was estimated as 40 % by angiography (a). IVUS evaluation demonstrates extensive plaque with 95 % area stenosis (b) (Courtesy of Boston Scientific)
Currently, the gold standard for identification of physiologically significant stenosis in the cath lab is fractional flow reserve assessment (FFR). Much of the data done with FFR has demonstrated ischemic lesions that were angiographically assessed as only 50 % stenosis, and non-ischemic lesions that appeared to be 80 % by angiography. This clearly demonstrates that angiography alone is not adequate in patients where the lesion is “intermediate.”
As with FFR, research with IVUS has been done in an attempt to stratify intermediate lesions as flow or non-flow limiting stenoses. However, as patients as well as coronary arteries come in all shapes and sizes the use of either MLA or MLD as a cut-point for significant/non-significant stenosis is problematic, as represented by the wide range of suggested values in the literature. In addition, there are multiple factors aside from lesion severity which contribute to overall functional significance such as length, eccentricity, and the myocardium which is subtended by the vessel. This is represented in the ACC/AHA 2011 PCI Guidelines in which FFR for evaluation of an intermediate stenosis receives an IIa (LOE A) recommendation and IVUS receives an IIb (LOE B) recommendation [6].
While FFR is currently considered by most the gold standard for invasive assessment of intermediate coronary lesions, significant work has been aimed at evaluating anatomic measurements by IVUS to predict significant coronary lesions. Early work with IVUS suggested a MLA cut point of ≥4.0 mm 2 for prediction of hemodynamically significant non-left main stenoses, in particular of proximal epicardial vessels. A MLA ≥4 mm2 had a diagnostic accuracy of 89 % in identifying a CFR of ≥2.0 [7]. In this same study only the MLA by IVUS and the lesion length were found to independently predict a CFR ≥2.0. In a study of 67 coronary lesions evaluated by both IVUS and stress myocardial perfusion imaging, a MLA of ≤4.0 mm2 had a sensitivity of 91 % and a specificity of 95 % for detection of functionally significant coronary artery stenosis [8]. Finally, in a study of 53 lesions with an angiographic stenosis of 40–70 % that were evaluated with both FFR and IVUS, a MLA of ≤4.0 mm2 had a sensitivity of 92 % and a specificity of 56 % in identifying an FFR of ≤0.75. In addition an MLD of ≤1.8, lesion length of >10 mm, and area stenosis >70 % were all strong predictors of ischemic FFR values [9].
While a MLA of ≤4.0 mm2 for non-left main stenosis has been used by many as a potentially reliable marker for determination of significance, there have been many other studies which have suggested different MLA values in addition to other vessel measurements in order to predict hemodynamically significant indeterminate lesions. In a group of 42 patients with 51 lesions that were evaluated by both FFR and IVUS the best determinant of an FFR <0.75 was an area stenosis of >60 % (sensitivity 92 %, specificity 89 %). In addition, if a patient had both an area stenosis >60 % and a MLA of <3.0 mm2, there was a 100 % agreement for the prediction of an ischemic lesion as defined as an FFR of <0.75 [10].In a more recent evaluation of IVUS to predict ischemia, 267 intermediate lesions in proximal and mid epicardial vessels were assessed by both IVUS and FFR with the goal of determining the best IVUS parameters and their diagnostic accuracy for functionally significant stenoses with an FFR <0.8. The best diagnostic accuracy was for the proximal LAD, where the best cutoff value to determine functional significance was an MLA of <3.0 mm2. For the mid LAD, a MLA of <2.75 mm2 was also found to have a reasonable diagnostic accuracy for ischemic lesions by FFR. However, IVUS measurements of the right coronary and circumflex arteries were not found to correlate well with prediction of ischemic lesions. In addition, the diagnostic accuracy for “large” (>3 mm) and small (<3 mm) vessels was also found to be poor indicating that lesion location (proximal or mid LAD and not the circumflex or right coronary) may also be a determinant of when to correctly use IVUS for evaluation of intermediate stenoses [11]. The diagnostic impact of the vessel being studied was also noted in a recent trial of 236 lesions evaluated by both IVUS and FFR. The independent determinants of FFR <0.80 were MLA, plaque burden, and left anterior descending artery location. In this study, the best cutoff value of the MLA to predict FFR <0.80 was 2.4 mm2 however the diagnostic accuracy was only 68 %. In the 117 lesions with a MLA ≥2.4 mm2, 96 % had a FFR ≥0.80. However only 37 % of lesions with a MLA <2.4 mm2 had an FFR <0.80 [12] suggesting that vessels with an MLA of ≥2.4 mm2 could be safely deferred while those <2.4 mm2 may need further evaluation with FFR.
The majority of studies investigating use of IVUS for intermediate non-left main stenoses evaluated proximal “large” caliber vessels >3 mm in diameter. However, in patients with small body habitus or diffuse coronary artery disease such as diabetics, many of the intermediate lesions occur in vessels with smaller luminal diameters. In the IDEAS (Intravascular Ultrasound-Derived Anatomic Criteria for Defining Functionally Significant Stenoses in Small Coronary Arteries) trial, 94 intermediate coronary lesions were evaluated by both IVUS and FFR. Definition of ischemic lesions in this trial was an FFR of <0.75 using intracoronary adenosine. The average reference vessel diameter was 2.72 mm and both proximal and mid epicardial vessels in all three major coronary arteries were evaluated. Over 40 % of lesions evaluated had a FFR <0.75. The three most important determinants of the FFR were the MLA, plaque burden, and lesion length. The best cutoff values for these determinants to discriminate a FFR of <0.75 were a MLA of ≤2.0 mm2, plaque burden ≥80 %, and a lesion length of ≥20 mm. Of the 22 lesions who were found to have all three IVUS determinants, 21 had an FFR of <0.75. When all three of these factors were combined the receiver operating characteristic was 0.94 and significantly increased compared with any of the IVUS characteristics alone. When using a FFR of <0.80 as the cutoff value, only MLA and lesion length were found to be of value in predicting functionally significant lesions, however the receiver operating characteristic curves remained high and similar to those for an FFR cutoff of <0.75 [13].
When taken together, the above data demonstrate a large amount of variability in specific cutoff values for determination of ischemic causing indeterminate coronary lesions. What is clear however is that measurements associated with significant coronary stenoses are MLA, lesion length, plaque burden, and area stenosis. While absolute cutoffs for these measurements is not feasible or reasonable for the vast majority of patients evaluated in the catheterization lab progressive reduction in MLA, longer lesions, high plaque burden, and increased area stenosis correlate with reductions in FFR particularly in proximal epicardial vessels, and ideally the left anterior descending. At this time FFR will likely remain the de facto stratifying test for intermediate lesions, however in patients where FFR is not feasible due to significant distal disease or intolerance of adenosine, IVUS remains an option for the interventional operator.
Assessment of Left Main Stenosis
Left main stenosis ≥50 % has been shown to have a poor long term outcome [14] while patients with mild or only moderate left main stenosis have a low 1 year event rate with medical management. In addition, premature bypass without physiologically significant stenosis can lead to premature graft closure [15]. Angiographic assessment of the left main is historically very difficult with high inter- and intra-observer variability due to significant foreshortening, ostial angulation, and streaming of contrast medium from the catheter tip in standard angiographic projections. Additionally, the typically diffuse nature of coronary artery disease in the left main limits the comparison of a diseased-free reference segment. With qualitative coronary angiography, the left main has been shown to be the least reproducible of any of the coronary segments. In an analysis of 810 angiograms from the Coronary Artery Surgery Study (CASS) of patients with left main stenosis, one angiographer reported a >50 % LMCA stenosis while a second angiographer reported no stenosis 19 % of the time in the same patients [16]. Further, multiple studies have noted that even with angiographically “normal” LMCA, up to 89 % of patients were found to have disease based upon IVUS evaluation [17].
FFR evaluation of intermediate lesions has been shown to accurately stratify patients that need revascularization from those that do not, with low event rates in those patients with non-physiologically significant FFR that are medically managed [18]. However, most patients with left main disease have additional coronary artery disease in their left anterior descending and circumflex arteries that may make performance of FFR inaccurate due to “protection” of the distal circulation from induced hyperemia.
The use of IVUS is uniquely suited for evaluation of ambiguous left main lesions in order to stratify patients that may need revascularization from those that can be safely deferred to medical management.
Before proceeding, it is important to recognize that angiographic evaluation of the left main is poor, particularly when an intermediate lesion is identified. Because of the poor prognosis associated with obstructive LMCA disease and the relatively normal survival of patient’s without obstructive LMCA disease, it is imperative that an additional modality other than angiography be used to stratify patients prior to revascularization. Because LMCA disease is most often encountered in association with disease in the remaining coronary tree, non-invasive stress tests may not accurately discriminate ischemia caused by the left main versus other coronary disease. In a group of 51 patients with angiographic intermediate (40–80 %) LMCA stenoses that were evaluated by FFR to determine functional significance (using cutoffs of both <0.75 and ≤0.80), 4 separate “experienced” interventional cardiologists blinded to FFR result were asked to classify the lesions as ‘significant,’ ‘not significant,’ or ‘unsure.’ The correlation with the FFR result for each reviewer was less than 50 % regardless of the FFR cutoff, meaning that over half the time the angiographic determination of significance was incorrect for each blinded reviewer. Furthermore, interobserver variability was large resulting in unanimously correct lesion classification in only 29 % of all cases [19]. Therefore, when making management decisions regarding the left main, angiography is often not enough.
The question however is what IVUS parameters should be used in order to determine functional significance? Because the left main is typically a short vessel, presence of disease in a single segment often predicts diffuse disease during IVUS interrogation. Because of this, it is difficult, if not impossible, to determine a “normal” vessel to use as a distal or proximal reference segment. In addition, positive and negative remodeling in the left main tends to be pronounced, again making identification of reference vessels difficult. For this reason, most of the studies evaluating IVUS parameters in comparison to FFR and SPECT have found MLA and MLD to be the two most predictive measurements correlating to ischemia.
The initial investigation of IVUS for stratification of intermediate or ambiguous LMCA lesions initially looked at patients with angiographically normal or minimally diseased LMCA in order to establish a “lower range of normal.” A total of 121 patients underwent IVUS evaluation of their LMCA and MLA were measured for all. These values were then plotted and a standard bell shaped, or Gaussian distribution, was plotted. The mean MLA for these patients with angiographically normal or minimally diseased LMCA was 16.25 mm2 with a standard deviation of 4.30 mm2. The “lower range of normal” was set at two standards deviations below the mean which was defined as a MLA of 7.5 mm2. Using this value as the cut point, 214 patients with intermediate angiographic left main lesions were then evaluated by IVUS. Of these patients, 39 % had an MLA of <7.5 mm2. Left main coronary artery revascularization was performed in 85.5 % (71 of 83) of patients with an MLA of <7.5 mm2 and deferred in 86.9 % (114 of 131) of patients with an MLA of ≥7.5 mm2. During long term follow-up (mean of 3.3 ± 2.0 years) there was no significant difference in major adverse cardiac events (target vessel revascularization, acute myocardial infarction, and death) [20]. This seminal investigation leads to the conclusion that deferring revascularization for patients with a MLA ≥7.5 mm 2 was safe.
While the previous study determined an MLA cutoff based upon the Gaussian distribution of the angiographically normal LMCA, the cutoff for ischemic producing lesions was initially investigated using an FFR of <0.75 as the gold standard. In 55 patients with angiographically ambiguous LMCA, FFR was initially obtained followed by performance of IVUS. The MLD and MLA were both found to strongly correlate with FFR. Compared with FFR, an MLD of 2.8 mm had the highest sensitivity and specificity (93 % and 98 % respectively) followed by an MLA of 5.9 mm2 (93 % and 95 % respectively) [21]. In a retrospective review of 115 “real world” patients found to have de novo angiographically intermediate LMCA stenosis evaluated by IVUS, a significant stenosis was defined as an MLA ≤6.0 mm 2 or a QCA diameter stenosis >50 % by angiography. A significant stenosis was seen in 44 % of lesions by IVUS but in only 13 % of lesions by QCA. In particular, only 36 % of ostial lesions were found to be significant by IVUS while QCA analysis of ostial lesions was the least accurate [22]. This study again highlights the inherent inaccuracies of angiographic evaluation alone. In addition, when using a MLA cut point of <6.0 mm2 to determine functional significance, less than half of patients evaluated by IVUS in this real world population were found to have significant disease suggesting that the majority of patients with angiographic ambiguous LMCA lesions were not severe by IVUS.
A prospective study to validate the use of a MLA of 6 mm2 as the cutoff value for revascularization was published in 2011. The LITRO (Prospective Application of Pre-Defined Intravascular Ultrasound Criteria for Assessment of Intermediate Left Main Coronary Artery Lesions) Study remains the largest prospective study evaluating an IVUS determined MLA to guide revascularization. A total of 354 patients were evaluated. Of the 168 patients with an MLA of <6 mm2, 90.5 % were revascularized, while 96 % of the 186 patients with MLA ≥6 mm2 were deferred to medical management. In the 2 year follow-up period survival free from cardiac was 97.7 % in the deferred group versus 94.5 % in the revascularized group (p = 0.5). Survival from death, myocardial infarction, and any revascularization was 87.3 % in the deferred group and 80.6 % in the revascularized group (p = 0.3). Importantly, in the 2 year follow-up period only 8 (4.4 %) patients in the deferred group required subsequent LMCA revascularization: in seven because of stable angina and in one after unstable angina [23]. Based upon this study, using a MLA cut point of <6 mm2 is safe, with little adverse events in patients deferred to medical management.
While an MLA of <6 mm2 and an MLA <2.8 mm are currently the accepted, albeit heavily debated, standards for IVUS evaluation of functionally significant LMCA stenoses and are recognized in the 2011 ACC/AHA guidelines [6], a study published in 2011 challenged these cutoffs as well. Using an FFR as the standard for determining physiological significance of LM stenosis, 55 patients (31 with stable angina and 24 with unstable angina) found to have an isolated LM lesion of 30–80 % angiographic diameter stenosis underwent IVUS and FFR. Using an FFR of <0.80 as the cutoff, ischemic FFR’s had significant correlation with the MLA, plaque burden, and angiographic length of the lesion. However, the MLA was the only independent determinant for FFR <0.80. The IVUS MLA that best predicted an FFR <0.80 was <4.8 mm2 with a sensitivity of 89 % and a specificity of 83 % [24]. Furthermore, very recent data suggests that an MLA of ≤4.5 mm 2 may also be a reasonable cutoff for prediction of physiologic stenosis as well [25].
It should not be surprising that there is no clear cutoff for IVUS derived LMCA cutoff values as vessels, like patients, come in a variety of shapes and sizes. What is clear based upon the current available data is that angiographic assessment of the LMCA often does not provide an accurate or reproducible assessment of the true degree of disease and should not be relied upon when the operator is making decisions regarding revascularization. When possible, FFR evaluation should be the initial adjunct diagnostic device for determination of physiologically significance. When this is not possible IVUS measurements, in particular the MLA and the MLD, have been shown to correlate with FFR although precise black and white cutoffs are not available nor are they reasonable to expect. LMCA stenoses with a MLA >6.0 mm2 have been shown to be safe for deferral to medical management in the only randomized, prospective trial available [23]. LMCA stenoses with an MLA of 4.8–6.0 mm2 represent a gray zone were some studies have suggested correlation with physiologically significant stenoses and others have not. Other factors to take into account in these situations would be lesion length, plaque burden ≥70 % [24], and MLD <2.8 mm which have all also been associated with significant FFR values although not to the degree as MLA.
IVUS Guided Bare Metal Stent Placement
While the restenosis rates improved significantly with the advent of bare metal stents compared with PTCA alone, high in-stent restenosis rates continue to be an issue. In a retrospective cohort of 1,706 patients undergoing bare metal stent placement the best predictors of binary restenosis during follow-up were longer stent length, smaller reference vessel MLD, and smaller stent CSA by IVUS. For every 1 mm2 increase in stent CSA there was a significant 19 % reduction in the risk for binary restenosis during follow-up [26]. Malapposition as well as underexpansion of stents has also been shown to contribute to restenosis and has led to the widespread adoption of the mantra of “bigger is better” and the adoption of high pressure balloon inflations during stent deployment.
The idea of IVUS guidance prior to intervention has many potential benefits to include adequate vessel sizing, assessment of degree of calcification in consideration for rotational atherectomy and evaluation of lesion length. Use of IVUS following intervention also has intuitive advantages, in particular allowing direct visualization of the stent to ensure that there is adequate apposition and expansion and it allows better assessment for complications such as distal and proximal edge dissections. This has led to a Class IIb recommendation for the use of IVUS for the guidance of coronary stent placement in the 2011 ACC/AHA PCI guidelines [6]. IVUS guidance of PCI has been shown to increase balloon and stent size, increase the length and number of stents used, and increase the MLD and stent CSA in comparison to angiographic guidance. The question of whether these changes lead to improved clinical outcomes however has resulted in mixed findings in many of the prospective studies evaluating IVUS guided versus angiographic guided bare metal stent implantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree