Introduction
SPECT and PET imaging require radiation sources and systems that can detect the radiation emanating from these sources. In Part I, the technical basis of PET-CT and SPECT-CT imaging systems was discussed. The other prerequisites for making these imaging examinations a clinical reality are discussed in Part II. These are the radiopharmaceuticals required for molecular imaging with PET and SPECT and the imaging protocols used to produce successful studies.
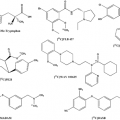
The emphasis in this book is on clinical applications of PET and SPECT. Currently, 90% of clinical PET uses FDG as a tracer. Still, the ensuing chapters are intended to give a somewhat broader perspective on the radiopharmaceuticals in clinical use and those with a definitive clinical potential. This book also discusses some uses of SPECT-CT. For SPECT-CT, the radiopharmaceuticals in use are more numerous, but again the most relevant radiopharmaceuticals that could benefit from SPECT-CT anatomic coregistration can be counted on two hands. Chapter 14 summarizes some of the important considerations regarding radiopharmaceuticals and their potentially desirable properties. Chapter 15 deals with the fluorinated PET compounds, which best lend themselves to distribution to satellite PET facilities. Chapter 16 discusses the perfusion radiopharmaceuticals that have established clinical utility, and Chapter 17 discusses the carbon 11 compounds, which have the obvious shortcoming of having a very short half-life. Finally, single photon compounds of clinical relevance for SPECT-CT are addressed in Chapter 18.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree