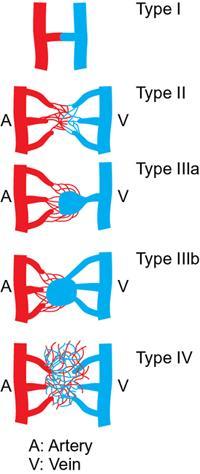
Kumar Shashi, Raja Shaikh Arteriovenous malformations (AVMs) are a heterogeneous group of high flow anomalies categorized by the International Society for the Study of Vascular Anomalies (ISSVA) as locally aggressive depending on their size and location. These are considered the most symptomatic and difficult vascular anomaly to treat, requiring multidisciplinary approach in most instances. Arteriovenous malformations comprise a group of congenital vascular anomalies with abnormal arteriovenous connections and absent intervening normal high-resistance capillary beds. Though congenital, they tend to grow concomitantly with the patient and factors influencing growth that include trauma, infection, hormonal status or haemorrhage. Less commonly they are seen as part of a broader syndrome such as Parkes–Weber syndrome, phosphatase and tensin (PTEN) homolog hamartoma syndrome and congenital lipomatous overgrowth, vascular malformations, epidermal nevi and skeletal deformities (CLOVES) syndrome. Most of these syndromes are secondary to sporadic gene somatic mutations such as MAPEK1 and HRAS. There is no sex predilection for sporadic AVMs. Extracranial AVMs can be solitary or regional. Symptoms vary and are largely dependent on location and extent of the AVM. The extremities, pelvis and the viscera are commonly involved. Extremity AVMs can manifest with venous hypertension, which can cause aneurysmal degeneration of draining veins, axial reflux, oedema and chronic inflammatory changes, especially in the lower extremities. Hyperdynamic circulation can cause arterial steal in limbs which can result in acral tissue loss and neurosensory loss. A high-output cardiac state with subsequent cardiopulmonary overload is a rare occurrence with chronic AVMs. Superficial lesions can present as compressible masses or with complications such as skin colour change or ulcers. Long-standing AVMs can exhibit a palpable thrill with dilated, tortuous and often aneurysmal draining veins, lipodermatosclerosis, venous oedema, lymphedema, bleeding and rarely signs and symptoms of cardiac overload. Internal and visceral AVMs present with vague general symptoms such as aches and throbbing pain, and bleeding diathesis depending on the location and size of the lesion. Osler–Weber–Rendu syndrome should be included in differential diagnosis when pulmonary AVMs are present. AVMs in the gastrointestinal tract can present with bleeding that can range from slow ooze to massive life-threatening haemorrhage again depending on the size, location and chronicity of the lesion. Portal venous hypertension, ascites and variceal bleeding can accompany larger, more widespread lesions. Schrodinger staging system is primarily used to clinically grade AVMs, but lacks angiographic morphological details to characterize the AVMs. To assist interventional treatment approaches, the Cho et al classification, and more recently Yakes and Baumgartner’s classification have been proposed. The Cho-Do classification categorized the AVMs into four groups based on the structure of feeding and draining vessels to the nidus and their predominant interventional approaches. The Yakes’ classification further classified lesions into groups which can be associated with treatment decisions. It emphasized not only the nidus but also the quantity and size of the draining veins (Fig. 2.19.1). The aim of imaging is primarily to characterize the angioarchitecture of AVM to aid appropriate management and to delineate local anatomical changes related to the lesion. At our institution, we rely on magnetic resonance imaging combined with dynamic magnetic resonance angiography for imaging of AVMs. Computed tomography (CT) with CT angiography offers high angiographic temporal resolution and optimal bone resolution. Cross-sectional imaging helps detect possible variant anatomy, foresee possible AVM related complications such as ischaemic necrosis, bleeding, infection and tissue injuries. Post processing imaging techniques such as 3D volume rendering and vessel marking with subsequent 3D models can assist interventionists to treatment approaches. Ultrasonography (USG) can be a helpful initial tool to diagnose a high flow malformation. AVMs characteristically lack definitive margins and have appearance of a hyper perfused and hypertrophied tissue with high velocity feeding arteries and ectatic arterialized draining veins. Phleboliths are unusual in AVMs. Limitations of USG include poor tissue penetration, inability to delineate the extent of lesion especially in complex and visceral AVMs. Colour Doppler imaging typically shows turbulent flow with aliasing. Ultrasound and colour doppler imaging is a very helpful and an inexpensive tool to follow-up AVMs during and after treatment. Catheter angiogram remains the gold standard for determining the flow dynamics and angio architecture in AVMs. It also helps decide the most suitable endovascular approach. Nowadays, diagnostic catheter angiograms are almost always performed as a part of endovascular treatment. Similar to other vascular malformations, management of AVMs requires multidisciplinary approach such as interventional radiology, plastic and reconstructive surgery and haematology services. Assistance from dermatologists, psychiatrists, social workers, physical therapy and rehabilitation specialists may also be needed in the long-term rehabilitation care of these patients. AVMs do not undergo spontaneous regression like haemangiomas and continue to grow at a physiological normal or rapid rate. They differ from neoplastic process as they possess normal endothelial turnover rates. Historically, treatment was reserved for symptomatic AVMs based on Schrodinger’s category. Stage 1 lesions were conservatively treated and observed with close follow-up, stage II lesions were treated when localized and stage III and IV lesions were always treated due to risk of haemorrhage and high output cardiac failure. Lee et al recommend treatment if the patient has one absolute indication or two relative indications (Table 2.19.1). At our institution we offer early endovascular treatment as soon as the diagnosis of AVM is established, even in the absence of any recognizable symptoms. This proactive approach has evolved from our experience that when left untreated, AVMs will continue to progress rapidly to more advanced and complicated stages limiting treatment options. We find it relatively easier to treat these lesions early in their natural course in order to curtail progression and prevent long term complications. Primary goals in AVM treatment are to control progression, prevent complications and disability, and more importantly, improve quality of life. Endovascular approaches aim at reducing the overall vascularity of the AVM by closing down inflow and outflow vessels and/or by closing the nidus using embolic and sclerosing agents. These agents are delivered via transarterial, transvenous, direct percutaneous approach or a by employing a combination of these techniques. Secondary endothelial inflammation elicited from these techniques also causes vascular thrombosis eventually blocking blood flow the nidus. These are mainly divided into
2.19: IR management of peripheral AVMs
Introduction
Etiology
Clinical presentation
Classification
Imaging
Treatment of arteriovenous malformations
Absolute Indications
Relative Indications
Haemorrhage
Disabling pain/progressive discomfort
Increasing or progressive risk of high-output cardiac failure
Functional disability or impairment affecting daily activities or quality of life
Chronic venous hypertension
Cosmetic deformity
Lesions located at a life/limb-threatening region
Vascular bone syndrome with limb length discrepancy or pelvic tilt causing scoliosis
Lesions threatening vital functions (typically head and neck CVMs)
Lesions located at a region with high risk of complications (e.g.: haemarthrosis, DVT)
Lesions with recurrent infection and sepsis
Principles of interventional radiology treatment of AVMs
Embolic agents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree