Knee
David Connell
Guilio Comin
INTRODUCTION
Ultrasound (US) is frequently used to guide percutaneous intervention in and around the knee joint and for the diagnosis of juxta-articular tendinopathies. Beyond this, some may consider ultrasound of the knee to be rather limited, with little else to contribute. Magnetic resonance imaging (MRI) is often favored as a multi-purpose tool for the evaluation of the knee, allowing an overall inspection of structures in and around the joint. However, ultrasound is often superior to MRI for the assessment of superficial structures such as tendons and is surprisingly capable for the assessment of deeper structures. As technology and operator experience have improved, the scope and application of diagnostic ultrasound have enlarged. Its utility has been demonstrated for the assessment of cartilage degeneration,1,2,3 chondrocalcinosis,4 anterior cruciate ligament rupture,5 meniscal injuries,6,7 impingement by intra-articular plicae,8 “snapping” syndromes,9 and other intra-articular pathology that are often considered the exclusive domain of MRI. Ultrasound has a major advantage over MRI when there has been a knee joint replacement, which usually results in substantial artifact, and has proven utility in diagnosis of postoperative complaints such as fibrous impingement10 and prosthetic loosening.11
Aside from its capability in tendon imaging due to excellent near-field spatial resolution and useful technical applications such as color Doppler and elastography, the main advantages of ultrasound are the ability to perform focussed examinations at sites of interest, during dynamic movement, and in varying positions.
CLINICAL ASSESSMENT
Ultrasound of the knee is predicated on targeted and dynamic scanning. When performed without consideration for the clinical features, this advantage is lost and ultrasound becomes less fruitful. Clinical assessment is an integral component of ultrasound examination of the knee and commences before meeting the patient, with an evaluation of the patient’s age. Children, young adults, and old adults are more likely to present with some specific pathologies than others. For example, anterior knee pain in a young child raises suspicion of Osgood-Schlatter disease, in an older child or a young adult of patellar tendinopathy, in a middle-aged adult of fat pad impingement or plica syndromes, and in an older adult of patellofemoral osteoarthritis. The patient’s stance and gait on entering the examination room give useful cues to the site and severity of pathology before any words have been spoken.
History Taking
The patient should be asked to recount current symptoms and concerns, recent and previous injuries, and previous surgery, and should be given time to do so without prompting or interruption, as valuable information can be brought to light that would otherwise be missed. The most important information to be gleaned includes:
Acute injury versus gradual development.
The nature of the injury or the activity(ies) that led to symptom development.
The type of symptom: pain, swelling, locking or snapping, stiffness, etc.
Region of symptoms. This can be grossly characterized as anterior, posterior, lateral, or medial, but patients are often able to localize pain with a fingertip, particularly in tendinopathies.
Exacerbating and relieving factors.
When pain is the predominant symptom, the type of pain: dull, sharp, burning, vague, etc.
The patient’s physical abilities and requirements: the type and “seriousness” of any sports played, the nature of their work, etc.
History of surgery and/or prior treatments.
Physical Examination
This should be performed in the ultrasound room with the lights fully up to permit proper observation of sometimes subtle clinical findings such as swelling, redness, bruising, surgical scars, or muscle wasting. Palpation should be performed for confirmation of tenderness or lump. It is often useful to mark regions of interest with a pen. The dynamic part of the clinical examination is usually best done with the ultrasound probe. A number of tests have
been described and validated. An in-depth discussion of their technique is beyond the scope of this chapter, but it may help sonologists to learn some of the more frequently used ones such as Apley, patella apprehension, Lachman, and anterior and posterior drawer tests.
been described and validated. An in-depth discussion of their technique is beyond the scope of this chapter, but it may help sonologists to learn some of the more frequently used ones such as Apley, patella apprehension, Lachman, and anterior and posterior drawer tests.
Clinical Correlation
Perhaps the most important reason for ensuring that a good history and examination have been performed is to allow a final consideration of the ultrasound findings in light of the clinical presentation. Do the ultrasound abnormalities explain all of the patient’s symptoms? If so, is the severity of the symptoms concordant with the severity of the ultrasound abnormality? If not, further investigation is required, perhaps with a different imaging modality, such as radiography or MRI, especially if there is suspicion of meniscal, bony, or chondral pathology.
ANATOMY
As ultrasound technology has improved, the anatomical knowledge required to perform and interpret ultrasound of the knee has increased. In the 1980s ultrasound involved a comparatively crude visualization of the juxta-articular tendons and assessment for joint effusion or Baker cyst. Current scanners allow visualization and characterization of intra-articular structures such as meniscus and cartilage, discrimination of tendons and capsule into component parts, and identification of fine nerve and vessel branches.
 Figure 7.1. Deep dissection diagram of the anterior knee demonstrating the articular surfaces and the menisci. |
As knowledge of knee mechanics and pathology has improved, there has been renewed interest in once obscure anatomical details, such as the vastus medialis insertion or the posterolateral capsular structures. The sonologist requires a wider and more intricate awareness of the normal location, function, and appearance of these structures.
Although a full description of this anatomy would require much more space than is available here, we will attempt to provide an overview and highlight the clinically and sonographically important points by breaking the anatomy down into its constituent parts:
Bone: Bone edges, periosteum, and tendon and ligament insertions.
Joint: Articular cartilage, meniscus and joint fluid.
Ligaments and Capsule
Tendons and Muscle
Bursae
Fat and fibrous layers
Nerves
Vessels
Bones
There are four or five bones at the knee joint: Femur, tibia, fibula, patella, and the inconsistently present fabella. The tibia and femur articulate at two surfaces or compartments: the medial femoral condyle with the medial tibial plateau, and the lateral femoral condyle with the lateral tibial plateau (Figs. 7.1 and 7.2). The medial articulation provides flexion and extension, while the
lateral articulation additionally provides some rotation and anterior/posterior translation.
lateral articulation additionally provides some rotation and anterior/posterior translation.
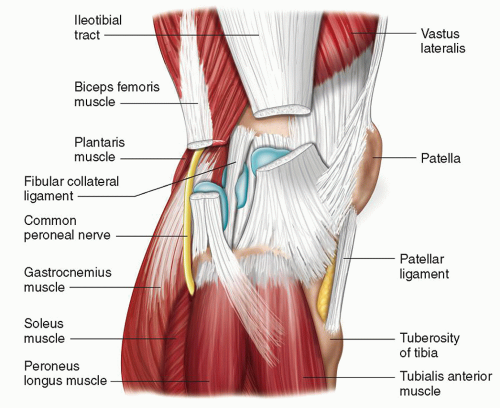
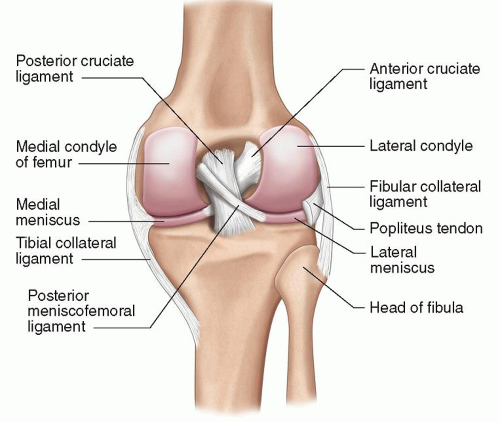 Figure 7.2. Deep dissection diagram of the posterior knee demonstrating the articular surfaces and the menisci. |
The patella articulates with the trochlea of the femur, a V-shaped groove, with medial and lateral facets of variable inclination that articulate with the medial and lateral patellar facets, respectively. A third and small “odd” patellar facet lies most medially and only articulates with the trochlea beyond 90 degrees of flexion.12
A bipartite (or tripartite) patella is a variant that results from failure of fusion of an accessory ossification center(s) with the remainder of the patella (Fig. 7.3). It occurs in 2% of the population, is more common in males than in females,13 and is typically, but not always, superolaterally located. As well as potentially being mistaken for a fracture, a bipartite patella occasionally becomes symptomatic when the fibrous union between the ossified parts is placed under stress. The so-called “dorsal defect of the patella” is a similar but incomplete bony defect in a superolateral position; it is unclear whether this is a related forme fruste of bipartite patella or an acquired lesion.
The base of the lateral tibial plateau articulates with the medial fibular head to form the proximal tibiofibular joint.
The fabella is a sesamoid bone that lies posteriorly, usually laterally, occasionally medially, and has a synovial articulation with the posterior femoral condyle. It is found, in varying states of ossification, in up to two-thirds of subjects,14 and has a potentially important role in stabilization of the posterior (and particularly posterolateral) capsule. When lateral, it has a complex relationship with the lateral gastrocnemius (Fig. 7.4) and plantaris tendons, and the oblique popliteal, arcuate, and fabellofibular ligaments (see below).
Bony prominences around the knee joint that are important and reliable landmarks for tendon insertion include the tibial tuberosity (for the patellar tendon) and Gerdy tubercle (for the iliotibial band [ITB]).
Joints
There are three consistent and distinct articulations at the knee. The largest are the tibiofemoral joint, which is further divided into medial and lateral compartments, and the patellofemoral joint. They share a common synovial cavity. Their articular surfaces are covered by hyaline cartilage of varying thickness. The medial and lateral menisci are interposed between the respective tibiofemoral articular surfaces. Menisci are fibrocartilaginous structures and act as shock absorbers and distributors of force, helping to maintain stability of the joint. They are attached centrally to the tibia by their anterior and posterior roots, as well as peripherally to the capsule. (Figs. 7.1 and 7.2)
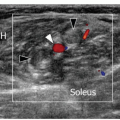
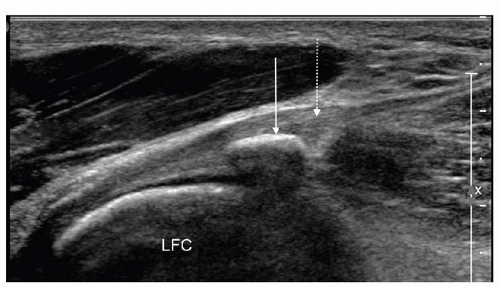 Figure 7.4. Longitudinal ultrasound image of the posterolateral capsule demonstrating the lateral gastrocnemius tendon with fabella (arrow). Lateral gastrocnemius tendon (dashed arrow). |
Plicae are folds of synovium within the knee joint. They are remnants of embryological structures and have three common locations in the knee:
Infrapatellar (ligamentum mucosum): Running obliquely in the intercondylar notch, in front of the anterior cruciate ligament (ACL).
Suprapatellar: Running vertically in the suprapatellar recess.
Mediopatellar: Running vertically at the medial aspect of the knee joint. When large, its free border can extend into the medial aspect of the patellofemoral joint, potentially resulting in symptomatic irritation.
The proximal tibiofibular joint is a separate, small joint and has its own synovial lining and hyaline cartilage surfaces.
Ligaments
The ligaments of the knee are classified as intra-articular or capsular.
The intra-articular ligaments are the cruciate ligaments and the variably present and thick meniscal and meniscofemoral ligaments.
The ACL originates from the posterolateral wall of the intercondylar notch and passes caudally, anteriorly, and medially to insert on the anterior tibial plateau, in front of the tibial spine (Figs. 7.1 and 7.2). It has two principal components, named for their position at the tibial insertion: The larger posterolateral bundle and the smaller anteromedial bundle. The former resists anterior translation in extension, and the latter resists anterior translation in flexion.
The posterior cruciate ligament (PCL) arises from the anteromedial wall of the intercondylar notch and passes caudally, posteriorly, and laterally to insert on the posterior aspect of the tibia, inferior to the tibial plateau (Figs. 7.1 and 7.2). It has two principal components: The larger anterolateral bundle and the smaller posteromedial bundle. The former resists posterior translation in flexion, and the latter resists posterior translation in extension.
The most anatomically consistent meniscal ligament is the transverse or meniscomeniscal ligament or intermeniscal ligament. The other meniscomeniscal ligaments are the posterior transverse ligament, connecting the posterior horns of the menisci, and the medial and lateral oblique ligaments, connecting the anterior and posterior horns. The meniscofemoral ligaments run from the posterior horn of the lateral meniscus to the posterolateral aspect of the medial femoral condyle, the ligament of Wrisberg posterior to the PCL and the ligament of Humphrey anterior to the PCL. There is no literature regarding ultrasound assessment of these structures, and they are not currently considered particularly clinically significant.
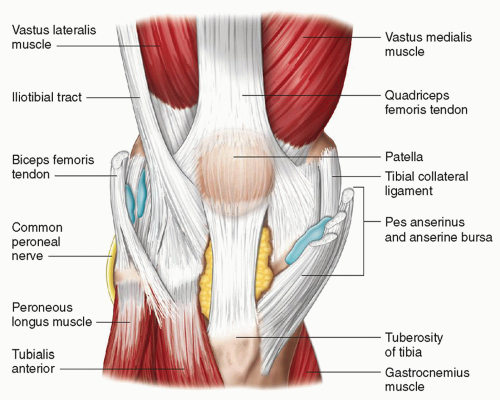
The capsular ligaments are focal condensations of the joint capsule and adjacent tissues. The largest are the medial and lateral collateral ligaments. They are thin elongated structures, are well defined, and have a lamellar appearance. The medial or tibial collateral ligament (MCL) arises from the medial femoral condyle and runs to insert on the medial edge of the tibia, well below the joint line (Fig. 7.5). It acts as a restraint to valgus angulation and external rotation. It is a discrete structure on ultrasound but blends imperceptibly at its anterior and
posterior borders with the adjacent superficial capsule. The superficial capsule at the anterior edge is composed of fibers from the MCL, fibers from the pes anserinus tendons (described below) and the so-called “crural” fascia. The superficial capsule at the posterior edge is composed of fibers from the MCL (the so-called posterior oblique ligament) and fibers from the semimembranosus tendon (described below) (Fig. 7.6). At the deep surface of the MCL are ligamentous attachments to the meniscus and the adjacent bones at the level of the joint, the meniscofemoral and meniscotibial (or coronary) ligaments, which are all well seen at ultrasound.15
posterior borders with the adjacent superficial capsule. The superficial capsule at the anterior edge is composed of fibers from the MCL, fibers from the pes anserinus tendons (described below) and the so-called “crural” fascia. The superficial capsule at the posterior edge is composed of fibers from the MCL (the so-called posterior oblique ligament) and fibers from the semimembranosus tendon (described below) (Fig. 7.6). At the deep surface of the MCL are ligamentous attachments to the meniscus and the adjacent bones at the level of the joint, the meniscofemoral and meniscotibial (or coronary) ligaments, which are all well seen at ultrasound.15
The lateral collateral ligament (LCL) arises from the lateral femoral epicondyle and runs posterolaterally to insert on the head of the fibula, often blending with the insertion of the biceps femoris (Fig. 7.7). Anteriorly, it may blend with the ITB insertion. The LCL primarily resists varus angulation and internal rotation and is well seen at ultrasound.
The capsuloligamentous anatomy at the posterior edge of the LCL (dubbed the posterolateral corner [PLC]) is both complex and clinically important. The PLC is primarily a restraint against varus angulation and external rotation. Identifying injuries to this area has important implications for surgical repair. Ultrasound is useful,16 and possibly superior to MRI, which is slightly handicapped by magic angle artifacts and the non-orthogonal orientation of the structures. The main components of the posterolateral corner are (Fig. 7.6):
Arcuate ligament: arises from the fibular tip and has a limb that passes posteriorly to form part of the posterior joint capsule, and a limb that passes superiorly to the lateral femoral condyle, paralleling the course of the LCL.
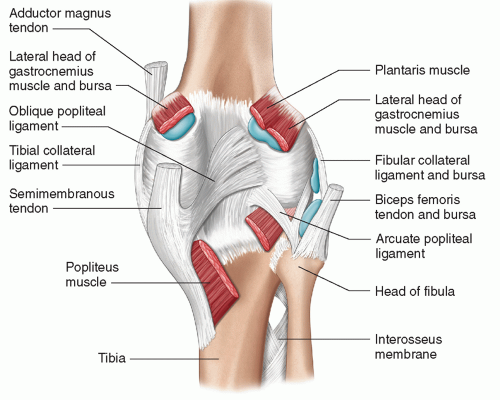
Figure 7.6. Superficial dissection diagram of the posterior knee showing posterior ligaments and bursa.
Popliteus tendon: runs from the popliteal groove of the lateral femoral condyle (an easy sonographic landmark), behind the joint and deep to the arcuate ligament, to the posteromedial tibia.
Fabellofibular ligament: passes from fabella to fibular head.
Popliteofibular ligament: passes from the popliteus to the fibular head.
Meniscopopliteal ligament: passes from the popliteus to the lateral meniscus. This structure may be important in facilitating the important role of popliteus in stabilizing the lateral meniscus.
All except the meniscopopliteal ligament are visible by ultrasound.17
The ligaments vary in size between individuals. In particular, the size of the arcuate ligament is inversely proportional to the size of the fabellofibular ligament, which in turn is variably important depending on the presence or absence of the fabella.18
Tendons
The tendons around the knee are classified into three groups: Anterior, posteromedial, and lateral.
Anteriorly, the quadriceps tendon is formed by the confluence of fibers from rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius. It has a trilaminar appearance, often appreciable on ultrasound with the superficial layer formed by fascicles from rectus femoris, the middle layer by fascicles from vastus medialis and
vastus lateralis, and the deep layer by fascicles from vastus intermedius (Fig. 7.8). The three layers are not completely fused and do have some degree of independent differential movement on knee extension. The quadriceps tendon inserts on the superior pole of the patella. The patellar tendon passes from the inferior pole of the patella to the tibial tubercle. Variable amounts of superficial quadriceps fibers run across the anterior surface of the patella and contribute to the patellar tendon. Strictly speaking, the non-quadriceps component of the patellar tendon is not a tendon as it connects bone with bone and should be more accurately described as a ligament.
vastus lateralis, and the deep layer by fascicles from vastus intermedius (Fig. 7.8). The three layers are not completely fused and do have some degree of independent differential movement on knee extension. The quadriceps tendon inserts on the superior pole of the patella. The patellar tendon passes from the inferior pole of the patella to the tibial tubercle. Variable amounts of superficial quadriceps fibers run across the anterior surface of the patella and contribute to the patellar tendon. Strictly speaking, the non-quadriceps component of the patellar tendon is not a tendon as it connects bone with bone and should be more accurately described as a ligament.
The patellar tendon is contiguous on either side with the medial and lateral patellar retinacula, which are important in ensuring appropriate patellar tracking. The medial retinaculum is formed by condensation of the joint capsule, medial fibers of the vastus medialis tendon (the so-called vastus medialis obliquus [VMO], and the medial patellofemoral ligament (MPFL), which arises from the adductor tubercle. The VMO and MPFL are important structures, both visible at ultrasound.19, 20, 21 The lateral retinaculum is formed by fibers from the vastus lateralis, ITB, and condensation of the joint capsule, and often has a bilaminar appearance
on ultrasound.22 The superficial layer is believed to be formed predominantly from the musculotendinous contributions, and the deep layer predominantly from the capsular contribution.
on ultrasound.22 The superficial layer is believed to be formed predominantly from the musculotendinous contributions, and the deep layer predominantly from the capsular contribution.
Posteromedially are the internal rotators and flexors (Fig. 7.5). The semimembranosus has a relatively wide insertion along the posterior and medial tibia near the joint line, and provides an important contribution to the posterior joint capsule (part of this contribution is named the oblique popliteal ligament (Fig. 7.6)), which is visible on ultrasound. The sartorius, gracilis, and semitendinosus tendons (or the “pes anserinus”) insert more anteriorly and more distally, at the transverse level of the tibial tuberosity (Fig. 7.5). The medial head of gastrocnemius originates above the posterior aspect of the medial femoral epicondyle and passes superficial to the joint.
Laterally, the biceps femoris inserts on the fibular head, blending here with the LCL insertion (Fig. 7.7), while further fibers variably extend to the adjacent tibia. The ITB, a condensation and continuation of the deep fascia of the thigh, is an important lateral stabilizer of the knee. It passes over the lateral femoral condyle, where it can become irritated by repetitive contact/friction, and inserts on Gerdy tubercle, which lies anteriorly, on the lateral edge of the lateral tibial plateau (Figs. 7.7 and 7.8). The lateral head of gastrocnemius and the plantaris arise from above the posterior aspect of the lateral femoral condyle (Fig. 7.7).
Bursae
Bursae are synovial-lined, fluid-filled sacs that lubricate movement between adjacent structures. They can become inflamed independently or fill with fluid due to communications with the knee joint, either congenital or acquired (degenerative/traumatic).
The most common bursae are:
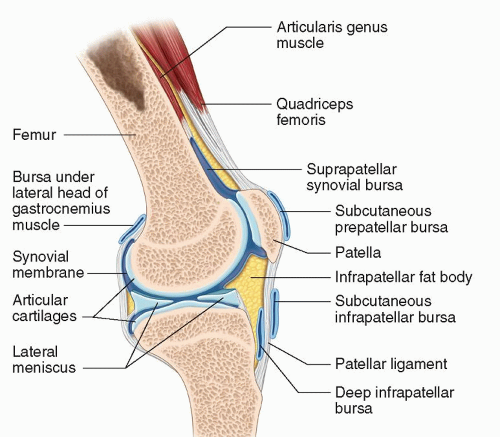
Anterior (Fig. 7.9)
Suprapatellar bursa (or recess): Deep to the quadriceps tendon and patella.
Prepatellar: Between patella and skin.
Deep infrapatellar: Between tibia and distal patellar tendon.
Subcutaneous infrapatellar bursa: Between patellar tendon and skin.
Pretibial: Between tibial tuberosity and skin.
Medial (Fig. 7.5)
Medial gastrocnemius: Between medial head of gastrocnemius and knee joint capsule.
Semimembranosus: Between semimembranosus and MCL.
Anserine bursa: Between MCL and pes anserinus.
Bursa between semimembranosus and tibia.
Lateral (Fig. 7.7)
Lateral gastrocnemius: Between the lateral head of gastrocnemius and knee joint capsule.
Fibular: Between LCL and biceps femoris.
Fibulopopliteal: Between LCL and popliteus tendon.
Subpopliteal: Between tendon of popliteus and lateral femoral condyle.
Vascular
The popliteal artery is the continuation of the femoral artery and passes posterior to the joint and the popliteus muscle. It branches into the anterior tibial artery and the tibioperoneal trunk at a variable level around the knee joint. The popliteal vein is the confluence of the anterior and posterior tibial veins. Anatomical variations are common. The most significant involves muscular entrapment of the popliteal artery by an abnormal course of the vessel or adjacent muscles.
The knee joint blood supply is from the various geniculate artery branches. The inferior lateral geniculate branch is an important and readily identifiable landmark at the posterolateral corner; it passes superficial to the popliteofibular and arcuate ligaments and deep to the LCL and fabellofibular ligament.
Nerves
The sciatic nerve runs along the posterior thigh and divides into its two main branches proximal to the knee joint. The tibial branch runs with the popliteal vessels directly posterior to the joint, while the common peroneal branch veers laterally to pass superficial to the distal biceps femoris and the fibular head. Both branches give
off further superficial sural cutaneous branches that supply the skin around the knee, whereas the common peroneal nerve divides into deep and superficial branches distal to the head of the fibula.
off further superficial sural cutaneous branches that supply the skin around the knee, whereas the common peroneal nerve divides into deep and superficial branches distal to the head of the fibula.
The femoral nerve runs anteromedially in the thigh, giving off multiple branches along the way. The most distal of these is the saphenous nerve, which runs deep along the posteromedial aspect of the knee, behind the sartorius tendon, and then becomes subcutaneous, piercing the fascia between the sartorius and gracilis tendons. The saphenous nerve gives off the infrapatellar branch, which supplies the skin of the anterior knee and is sometimes injured at surgery.
All of these nerves can be visualized by ultrasound, particularly when pathological.
TECHNIQUE
Several transducers are required to perform a scan of the whole knee. Superficial structures should be examined with a high-frequency linear transducer. Deeper structures need lower frequency transducers; for example, a 3.5 MHz transducer may be required to examine the deepest aspects of the popliteal fossa, such as the PCL origin.
The core controls of gain, time gain compensation (TGC), depth, and focus should be continually adjusted to optimize the ultrasound image. Harmonic imaging provides improved contrast resolution at the cost of poorer spatial resolution, and is useful when scanning fluid-filled structures such as ganglia, joint effusions, collections, or fluid-filled muscle or meniscal tears. The fluid appears more obviously anechoic, and any true echoes within the fluid will be highlighted.
Compound imaging improves images by obtaining echoes from areas that previously were anechoic; for example, when there is anisotropy or acoustic shadowing from calcifications or wall edges, compound imaging can fill in these areas. The downside is that sometimes identification of these artifacts is required to make a diagnosis, for example, dense acoustic shadowing distal to foreign bodies and calcification or acoustic enhancement to confirm that a structure is fluid-filled.
Color Doppler imaging should be used since hyperemia is a sensitive marker for inflammation and tendinopathy. Accurate assessment requires skilled use of the Doppler controls. When examining superficial structures with Doppler, it may be worth switching transducers as lower frequency transducers show low-velocity blood flow better than high-frequency transducers. The pulse repetition frequency (PRF) and wall filter should be set low and the Doppler gain setting below the level of saturation. The knee should be extended to relax the quadriceps and patellar tendons, and only very light transducer pressure should be employed or vascularity may be obliterated. Comparison with the opposite side may help.
A complete knee scan that examines all four anatomical quadrants is rarely needed. Most ultrasound examinations of the knee are targeted to answer specific clinical questions, but a systematic approach to each quadrant is desirable so that all relevant anatomy is scanned. This text describes a uniform approach to positioning and examination that should be adapted to meet the needs of the individual patient.
Anterior Knee Assessment
Ultrasound assessment of the extensor mechanism can be considered as a three-part examination, starting superiorly and finishing inferiorly. First, the suprapatellar structures of the quadriceps tendon, suprapatellar recess and the trochlea of the femur are examined; second, the patella and associated structures including the retinacula; and third, the patella tendon and bursae (e.g., prepatellar and infrapatellar bursa).
The anterior knee also includes the slightly off-midline structures of the ITB and the pes anserine insertion.
The examination starts with a higher frequency transducer. Two patient positions are used:
1. The examination commences with the knee in 30 degrees of flexion (Fig. 7.10) to ensure that the quadriceps and patellar tendons are stretched and the risk of anisotropy artifact reduced. Careful scanning in longitudinal and transverse planes should be performed. For the quadriceps, this is from the distal third of the thigh to the insertion on the patella. Due to the large area to be covered, this is achieved by multiple sweeps, scanning each of the four muscles
separately into their tendon and down to the patellar insertion. The contours of the patella and the retinaculum should be included in the examination to look for retinacular thickening or tears.
separately into their tendon and down to the patellar insertion. The contours of the patella and the retinaculum should be included in the examination to look for retinacular thickening or tears.
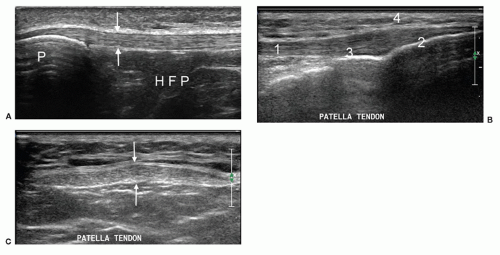
The patellar tendon is scanned from its origin on the patella to its insertion on the tibial tuberosity in longitudinal and transverse planes (Fig. 7.11 A-C). As pathology here can be subtle, the contralateral tendon should be scanned for comparison. The structures seen superficial and deep to the tendon should also be assessed. Some may only present when pathological, such as the prepatellar or pretibial bursae. The infrapatellar fat pad of Hoffa (Fig. 7.11A) lies deep to the patellar tendon. There is normally clear demarcation between the lower level echoes of fat lobules and the hyperechoic interconnecting fascia. The remaining infrapatellar structures of the ITB and pes anserinus should also be assessed.
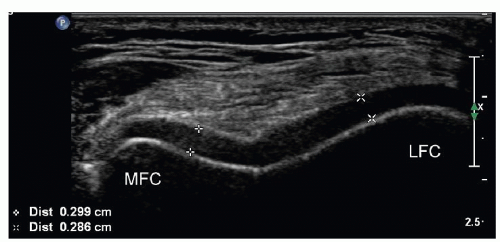
Parts of the femoral cartilage can be examined if the knee is positioned in full flexion (Fig. 7.12). Changes in its usual hypoechoic echogenicity should be noted. Comparison with the opposite knee helps to assess thickness and contour.
2. With the knee fully extended and relaxed, the tendons and fat pads are assessed with color Doppler.
Tip:
Examine for vascularity in the patellar tendon with the knee extended and relaxed. This is not the optimum position for B-mode assessment as the tendon is not fully stretched and anisotropy artifact may occur.
The suprapatellar space can be examined for excess fluid in varying degrees of knee flexion to increase sensitivity for knee joint fluid.23
Tip:
Knee joint fluid is best detected by asking the patient to elevate the ankle with the knee extended. This tenses the knee joint and pushes any excess joint fluid into the supra patellar space.
While these are the minimum two positions required for the anterior knee, scanning in other degrees of flexion may produce more fluid in or around structures or open up defects/tears in retinacula, ligaments, or tendons.)
Tip:
If the patient is mobile, consider scanning while the patient sits on the edge of the examination couch with the foot or calf resting on the knee of the sonologist who sits opposite the patient. This allows access to all four quadrants of the knee without the patient having to move, and achieves good venous distension and visualization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree