Nerves
Carlo Martinoli
Alberto Tagliafico
Ian Beggs
INTRODUCTION
Clinical assessment, supplemented by neurophysiological testing, remains fundamental to the diagnosis of peripheral nerve lesions, and can be supplemented by imaging. Radiographs and computed tomography (CT) are of limited value, although CT shows bony or osteophytic encroachment on fibro-osseous tunnels. Magnetic resonance (MR) imaging and ultrasound are both capable of demonstrating peripheral nerves. Conventional MR imaging is widely available. Specialized techniques such as MR neurography, including diffusion tensor imaging,1,2 are particularly useful centrally and in deep nerves that are inaccessible to ultrasound, but are not widely available. Ultrasound is ideally suited for assessment of peripheral nerves. Although it is widely available, relatively cheap, assesses long segments of nerves quickly, and permits dynamic examination,2 careful technique and good anatomical knowledge are essential.
ANATOMY AND ULTRASOUND TECHNIQUE
Peripheral nerves are composed of myelinated and nonmyelinated axons that are bathed in endoneurial fluid. Axons are grouped together in fascicles that are surrounded by perineurium consisting of perineurial cells and collagen. Fascicles are embedded in connective tissue, the interfascicular epineurium, which contains vessels and elastic fibers. The outer layers of epineurium envelop the fascicles and form the nerve sheath or epineurial epineurium.3 The size and number of fascicles vary between and within nerves.
Ultrasound scans of peripheral nerves correlate well with nerve structure (Fig. 12.1). Long-axis scans show narrow, elongated structures (Fig. 1.16) with a fascicular appearance due to alternating hypoechoic and hyperechoic bands. In short-axis scans, nerves exhibit a stippled, honeycomb-like appearance (Fig. 12.1) due to small hypoechoic rounded foci embedded in a hyperechoic background. The hypoechoic areas correspond to fascicles. The echogenic tissue is due to epineurium.4 The outer boundaries of nerves are usually undefined due to the similar hyperechoic appearances of the outer epineurium and the surrounding perineural fat.
In general, nerves are soft and flexible. They may change shape from oval to round, depending on the width of the anatomic passageways within which they run and the echogenicity of the perineural structures that lie in contact with them. Nerves are mobile. Light transducer pressure or patient movement may cause a nerve to slide over the surface of an artery, tendon, or muscle. Compared to tendons, nerves are less echogenic and less susceptible to anisotropy and the ultrasound beam does not have to be perpendicular to assess them.4 Nerves move less than tendons. Alternating flexion and extension of the fingers, for example, show long excursions of the flexor tendons at the wrist but relatively short excursions of the median nerve. Where nerves cross narrow osteofibrous tunnels around joints, they may assume a more homogeneous hypoechoic appearance due to tighter packing of fascicles, and the outer epineurium may be slightly thickened.
Careful scanning technique based on precise knowledge of nerve position and analysis of anatomic relationships is necessary. A high-frequency (center frequency >10 MHz) linear array broadband transducer and copious gel are required. Ultrasound is initially best performed by scanning a nerve in short axis. Bony landmarks and structures such as tendons and vessels are easily identified, and help to locate the nerve. Vessels are particularly useful landmarks, but not all nerves run alongside a vessel—the median nerve at the wrist and the sciatic nerve being prominent examples. Once the nerve is identified, the transducer is swept repeatedly proximally and distally, using an “elevator” technique, to examine long segments of nerve rapidly, looking for alterations in size, shape, and texture of the nerve and abnormalities in adjacent structures. The transducer is then rotated through 90 degrees and the nerve is examined in its long axis; this is particularly useful when looking for alterations in nerve caliber. Dynamic examination may show alterations in nerve position and shape as muscles contract or joints move. Although nerves can be displayed in the limbs due to their superficial position and absence of intervening bone, not all nerves can be shown with ultrasound. Cranial nerves; nerve roots exiting the
dorsal, lumbar, and sacral spine; the sympathetic chains; and the splanchnic nerves in the abdomen cannot be visualized because they are deep or obscured by interposed bony structures or bowel.
dorsal, lumbar, and sacral spine; the sympathetic chains; and the splanchnic nerves in the abdomen cannot be visualized because they are deep or obscured by interposed bony structures or bowel.
ANATOMICAL VARIANTS
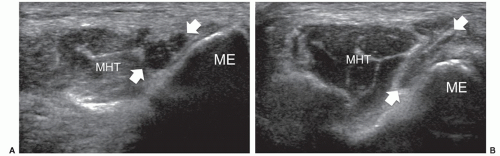
Most anatomical variants affecting peripheral nerves are not of clinical significance but some variants may result in symptoms or be important for surgical planning. The most frequently encountered variant is the bifid median nerve at the wrist, with or without a persistent median artery.5,6,7 In the bifid median nerve, two bundles of fascicles enter the carpal tunnel side by side (Fig. 12.2A). The persistent median artery is an accessory artery that arises from the ulnar artery at the proximal forearm and accompanies the median nerve along its course throughout the forearm and carpal tunnel. It can be associated with a bifid or normal nerve (Fig. 12.2B).
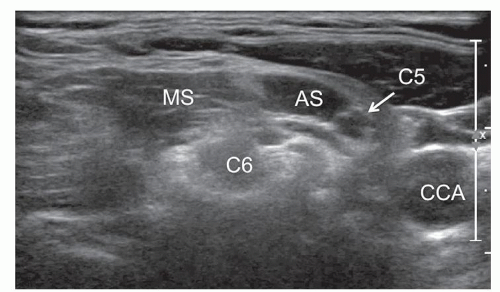
Nerves may also have an anomalous course, for example, at the brachial plexus level when the C5 and/or C6 roots do not cross between the scalenus anterior and medius but penetrate the anterior scalene. The anomalous course of the C5 root in front of the anterior scalene muscle occurs in 3% to 3.2% of cases (Fig. 12.3).8
Extrinsic abnormalities along the course of a nerve may predispose to nerve compression. The supracondylar
process on the anteromedial surface of the distal humerus is a rare anatomical variant consisting of a beak-like bone projection with a large base, located about 7 cm proximal to the medial epicondyle and directed distally. It may be associated with the Struthers ligament that inserts on the medial epicondyle to form an osteofibrous tunnel. The median nerve and the brachial artery pass through this tunnel and may be compressed, producing a syndrome of pain and numbness in the hand, exacerbated by elbow extension and forearm pronation, and flexor weakness.
process on the anteromedial surface of the distal humerus is a rare anatomical variant consisting of a beak-like bone projection with a large base, located about 7 cm proximal to the medial epicondyle and directed distally. It may be associated with the Struthers ligament that inserts on the medial epicondyle to form an osteofibrous tunnel. The median nerve and the brachial artery pass through this tunnel and may be compressed, producing a syndrome of pain and numbness in the hand, exacerbated by elbow extension and forearm pronation, and flexor weakness.
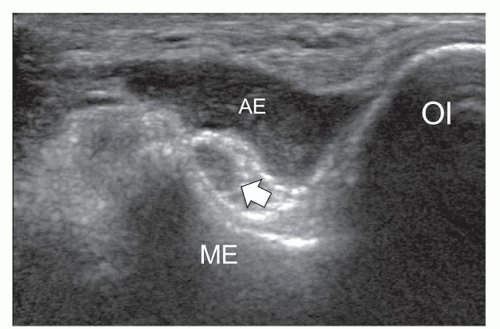
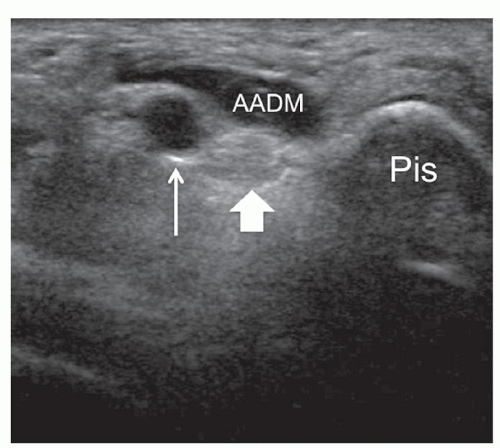
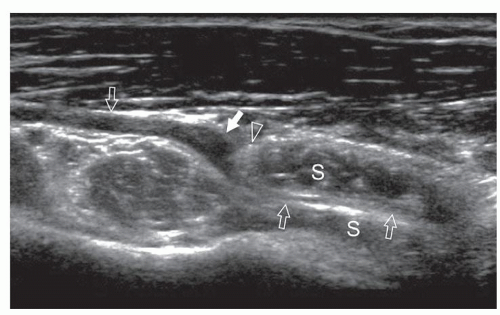
Several anomalous muscles in the upper and lower extremity may predispose to nerve entrapment. The chondroepitrochlearis is a very long, narrow slip arising from the inferolateral margin of the sternocostal head of the pectoralis major. It inserts into the medial intermuscular septum or the medial epicondyle after crossing the axilla and the arm9 and may compress the ulnar nerve. The anconeus epitrochlearis is a small aberrant muscle on the posteromedial elbow (Fig. 12.4). It extends from the medial epicondyle to the olecranon with a transverse course replacing the Osborne retinaculum.10 During elbow flexion, ultrasound can show dynamic impingement of the muscle on the underlying ulnar nerve at the epitrochlear groove. The Gantzer muscle is an accessory head of the flexor pollicis longus that bridges the anterior interosseous nerve at the proximal forearm possibly predisposing it to local entrapment.11 In reversed palmaris longus, the muscle is located distally and the tendon proximally. The excess muscle tissue immediately superficial to the flexor digitorum superficialis may predispose to carpal or Guyon tunnel entrapment, and ultrasound can demonstrate dynamic impingement of the muscle on the median nerve during active contraction.12 The palmaris profundus arises from the flexor digitorum superficialis or the distal radius and ulna and inserts into the deep surface of the distal flexor retinaculum after traversing the carpal tunnel. Its tendon may compress the median nerve.13 In some instances, the nerve and the tendon may adhere or share a common sheath (musculus concomitants nervi mediani). The accessory abductor digiti minimi (AADM) is a common fleshy band forming the roof of the Guyon tunnel, anterior to the neurovascular bundle (Fig. 12.5). If hypertrophied, it may cause ulnar neuropathy by squeezing the ulnar nerve against the pisohamate ligament during contraction.14
In the ankle, the flexor digitorum accessorius longus crosses the tarsal tunnel deep to the flexor retinaculum. The close relationship with the tibial nerve may explain the relatively high prevalence (12.2%) of this accessory muscle in patients with tarsal tunnel syndrome.15
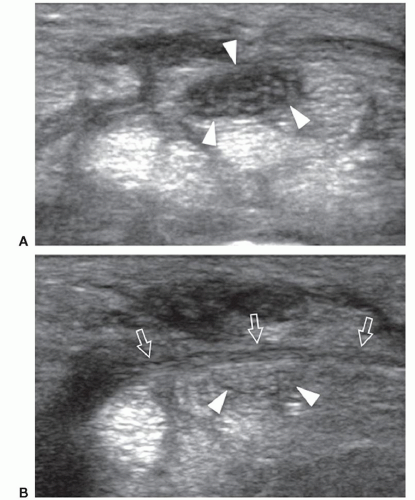
Congenital absence of retinacula at osteofibrous tunnels may cause nerve instability during joint motion. In ulnar nerve instability at the elbow, dynamic scanning during elbow flexion can show anterior dislocation of the nerve over the medial epicondyle (Fig. 12.6) (16). This may be associated with dislocation of a prominent medial head of the triceps muscle (snapping triceps syndrome).16
Tip:
High-resolution ultrasound provides rapid examination of peripheral nerves of the upper and lower extremities.
Peripheral nerves have a well-defined fascicular echotexture made of clusters of hypoechoic fascicles embedded in a hyperechoic epineurium.
Sweep the transducer proximally and distally (“elevator” technique) in short axis along the nerve to examine long segments rapidly.
Anatomical variants of nerves and their surroundings (e.g., accessory muscles) are reliably recognized with ultrasound.
COMPRESSIVE NEUROPATHIES
Entrapment neuropathies are usually the result of chronic or dynamic compression of nerves in fibro-osseous or fibromuscular tunnels. Tunnels have rigid walls and accessory muscles, ganglia, osteophytes and other space-occupying lesions elevate pressure in the tunnel and compress the nerve. Compression at other sites, for example, by scarring, is less common. Chronic compression results in ischemia, Wallerian degeneration, and fibrosis of the nerve and motor and sensory disturbances. Identifying the site of the lesion depends on precise anatomical knowledge. Neurophysiological studies are often helpful but have a significant false-negative rate. Magnetic resonance imaging can show entrapment and downstream effects such as denervation changes in muscle.17,18,19 Although possibly less sensitive to denervation changes than MR imaging, ultrasound has high spatial resolution and can provide excellent detail of tunnels and their contents, including nerve morphology.
On the basis of ultrasound assessment, nerve compressive syndromes can be grouped into three main classes.
Class-1 includes large nerves (e.g., median, ulnar, peroneal, and tibial) that are easily imaged by ultrasound at the compression site using conventional transducers (probe frequency up to 13 MHz). Assessment is based on pattern recognition and, for the carpal and cubital tunnels, nerve cross-sectional area (CSA) with diagnostic performance nearly equivalent to MR imaging.
Class-2 includes small (caliber <2 mm) nerves (e.g., posterior and anterior interosseous, musculocutaneous, sural, and distal divisional branches of large nerves) that require high-end equipment and higher frequency probes (up to 18 MHz). The diagnosis relies exclusively on pattern recognition because the nerves are too small for CSA measurements. There is no objective evidence comparing the performance of ultrasound and MR imaging in depicting pathology of these nerves, but in our opinion, ultrasound with an appropriate transducer in experienced hands often seems to be superior to MR imaging. Compared with MR imaging, the main advantage of ultrasound is its ability to distinguish distal nerves from vessels. The frequent absence of denervation changes, because many distal nerve branches are purely sensory, is a major disadvantage of MR imaging.
Class-3 includes small (e.g., inferior calcaneal) and large (e.g., femoral and sciatic in their intrapelvic course) nerves that are poorly visible or nondetectable with ultrasound due to a deep course or intervening bone. Although these nerves cannot be directly imaged, ultrasound can suggest a nerve problem by showing denervation changes in muscles such as loss of bulk and increased echogenicity. Ultrasound is not as accurate as MR imaging in differentiating early denervation changes of intramuscular extracellular edema from fatty atrophy.20
Regardless of the entrapment site, the ultrasound signs of compressive neuropathy are characteristic. The nerve appears flattened at the compression point and swollen proximally.21 The transition between flattened and swollen segments is abrupt (notch sign). Ultrasound accurately identifies the exact level of compression based on these features. Intraneural edema and venous congestion lead to nerve enlargement in the early phases of compression.22 The increased water content correlates with axon loss (axonotmesis),23 and there is a positive correlation between the nerve CSA and the severity of electromyography (EMG) findings.24 Nerve echotexture may appear uniformly hypoechoic with loss of the fascicular pattern due to swelling of the fascicles and reduced echogenicity of the epineurium. These changes are more profound in severe long-standing compression.21 Occasionally, intraneural hyperemia can be appreciated at Doppler imaging.21,25 At the carpal tunnel this seems to correlate with disease severity and to be a good predictor of median nerve entrapment.25,26 Irreversible intraneural fibrosis may occur in long-standing compression. In contrast to early disease, nerves with fibrotic changes tend to remain swollen after decompressive surgery and tend to show poor functional improvement.27
In nerve entrapment syndromes, the measurement that has to be taken is the CSA for class-1 nerves or the maximum cross-sectional diameter for class-2 nerves. The CSA should be calculated where the nerve is maximally enlarged and the histopathologic changes are most severe, either by the indirect method using calipers and application of the ellipse formula (transverse diameter × anteroposterior diameter × π/4), or the direct method, based on manual tracing and automated calculation of the area.28,29 Both methods have high reproducibility and short learning periods.30 The CSA measurement should be obtained from the outer margin of hypoechoic fascicles. The echogenic rim surrounding the fascicles due to the outer epineurial sheath has to be excluded because it may not be clearly distinguishable from the external perineural fat.29 Probe position can affect the variability of CSA measurements: The ultrasound beam should always be directed perpendicular to the long axis of the nerve (even if the nerve assumes a curved or oblique course). Optimal probe orientation for measurements can be defined dynamically by tilting the probe over the nerve or inducing slight changes in the joint position. When setting cutoff values for nerve CSA, gender, weight, body mass index, and race should be taken into account as possible confounders.31
Several sites of nerve entrapment are amenable to ultrasound examination in the upper and lower extremities. The most common conditions are described below using a regional approach.
Shoulder
The suprascapular nerve is a purely motor nerve arising from the upper trunk of the brachial plexus (C5-C6). It descends through the suprascapular foramen
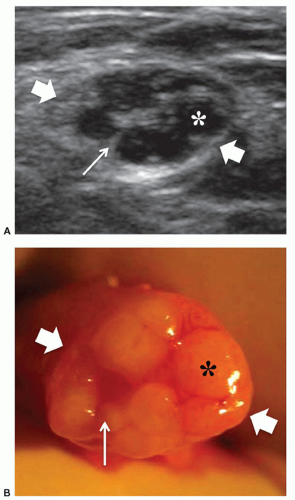
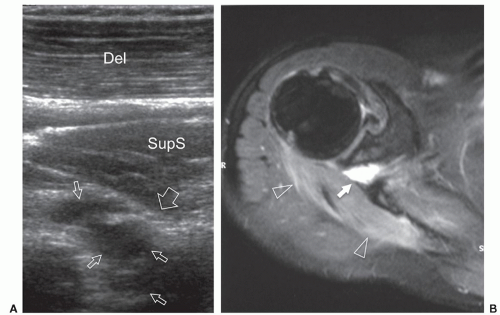
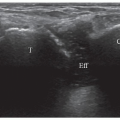
to the supraspinous fossa and the spinoglenoid notch. Suprascapular neuropathy leads to chronic shoulder pain and weakness. It may be secondary to constriction of the nerve at the suprascapular or spinoglenoid notches by stretching injuries, ligament abnormalities, overuse, or space-occupying lesions. When the nerve is trapped in the supraspinous fossa, the supraspinatus and infraspinatus muscles may undergo denervation changes; when it is compressed at the spinoglenoid notch, denervation is limited to infraspinatus. Paralabral cysts are the leading cause of suprascapular nerve compression32,33 and are usually associated with tears of the posterior glenoid labrum (from 8 to 11 o’clock positions). The cyst extends into the spinoglenoid and/or suprascapular notches deep to the muscles bellies, and may cause muscle denervation (Fig. 12.7). Large cysts (>3 cm) are most often associated with compressive neuropathy. Paralabral cysts are round or oval hypoechoic lesions with well-defined margins. They are relatively fixed in location and shape during arm movements. Due to their deep location, even large cysts may be missed during standard ultrasound examination of the rotator cuff. The continuity of the cyst with a defect in the posterior labrum may be revealed with ultrasound. Percutaneous needle aspiration to decompress the nerve can be attempted under ultrasound guidance.34
to the supraspinous fossa and the spinoglenoid notch. Suprascapular neuropathy leads to chronic shoulder pain and weakness. It may be secondary to constriction of the nerve at the suprascapular or spinoglenoid notches by stretching injuries, ligament abnormalities, overuse, or space-occupying lesions. When the nerve is trapped in the supraspinous fossa, the supraspinatus and infraspinatus muscles may undergo denervation changes; when it is compressed at the spinoglenoid notch, denervation is limited to infraspinatus. Paralabral cysts are the leading cause of suprascapular nerve compression32,33 and are usually associated with tears of the posterior glenoid labrum (from 8 to 11 o’clock positions). The cyst extends into the spinoglenoid and/or suprascapular notches deep to the muscles bellies, and may cause muscle denervation (Fig. 12.7). Large cysts (>3 cm) are most often associated with compressive neuropathy. Paralabral cysts are round or oval hypoechoic lesions with well-defined margins. They are relatively fixed in location and shape during arm movements. Due to their deep location, even large cysts may be missed during standard ultrasound examination of the rotator cuff. The continuity of the cyst with a defect in the posterior labrum may be revealed with ultrasound. Percutaneous needle aspiration to decompress the nerve can be attempted under ultrasound guidance.34
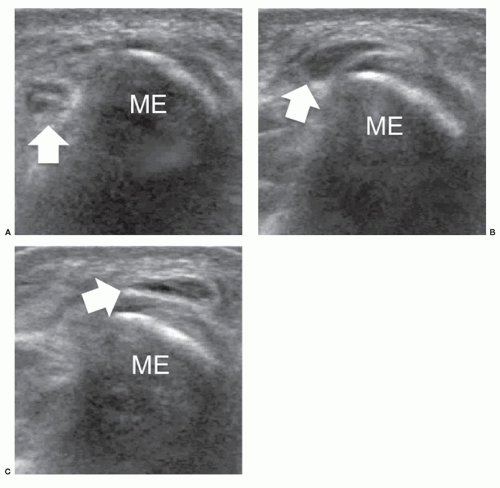
The axillary nerve originates from the posterior cord of the brachial plexus (C5-C6) near the coracoid. It proceeds along the inferolateral border of the subscapularis muscle to curve inferior to the glenohumeral joint capsule and pass to the posterior aspect of the arm with the posterior circumflex artery through the quadrilateral space.35 The axillary nerve has two terminal branches, anterior and posterior. The anterior branch innervates the anterior part of deltoid, the posterior supplies teres minor and the posterior deltoid. Ultrasound detection of the axillary nerve is challenging due to its small size and deep course. With arm elevation, the axillary neurovascular bundle can be imaged as it runs across the quadrilateral space close to the inferior aspect of the glenohumeral joint. Axillary neuropathy is caused by stretching injuries or stenosis of the quadrilateral space by fractures, fibrous bands, or inferior (from 9 to 7 o’clock positions) paraglenoid cysts.36 Teres minor atrophy can be assessed by comparing the size of the muscle with the adjacent infraspinatus (Fig. 12.8). Atrophy of deltoid is confirmed by reduced thickness relative to the opposite side.37 Ultrasound is able to demonstrate paralabral cysts extending from the inferior aspect of the glenoid in association with a tear of the inferior labrum.
Elbow
At the medial elbow, the ulnar nerve courses in the condylar groove, a space delimited by the olecranon and the medial epicondyle and bridged by the cubital tunnel retinaculum (Osborne fascia). The nerve then enters the proper cubital tunnel, a narrow passage between the
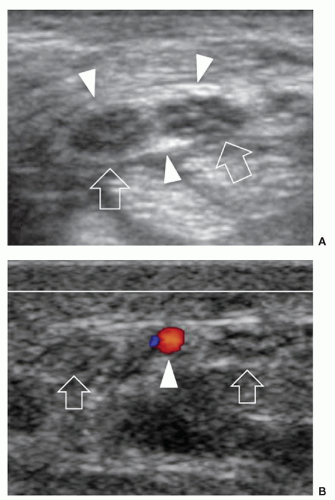
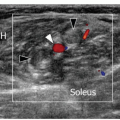
ulnar and humeral heads of the flexor carpi ulnaris covered by the arcuate ligament, a distal expansion of the Osborne fascia. Ulnar nerve compression may occur at the condylar groove (proximal tunnel) or at the edge of the arcuate ligament (distal tunnel) due to bony abnormalities (e.g., cubitus valgus, deformities from previous fractures, medial osteophytes, and loose bodies), thickening of the floor of the tunnel (the joint capsule and the posterior band of the medial collateral ligament), and space-occupying masses (ganglia and accessory muscles, i.e., anconeus epitrochlearis). In early or mild cases, the clinical diagnosis of cubital tunnel syndrome may be difficult, and electrophysiology has a low sensitivity, ranging from 37% to 86%.38 In cubital tunnel syndrome, ulnar nerve CSA >10 mm2 is generally accepted as abnormal with sensitivity as high as 95% to 100%,39 but there is poor correlation between ulnar nerve size and electrophysiology. Ultrasound demonstrates a fusiform hypoechoic swelling of the ulnar nerve with loss of the fascicular pattern.40 Ultrasound examination during elbow flexion should always be obtained to identify dynamic nerve impingement (e.g., prominent medial head of triceps) (Fig. 12.9).
ulnar and humeral heads of the flexor carpi ulnaris covered by the arcuate ligament, a distal expansion of the Osborne fascia. Ulnar nerve compression may occur at the condylar groove (proximal tunnel) or at the edge of the arcuate ligament (distal tunnel) due to bony abnormalities (e.g., cubitus valgus, deformities from previous fractures, medial osteophytes, and loose bodies), thickening of the floor of the tunnel (the joint capsule and the posterior band of the medial collateral ligament), and space-occupying masses (ganglia and accessory muscles, i.e., anconeus epitrochlearis). In early or mild cases, the clinical diagnosis of cubital tunnel syndrome may be difficult, and electrophysiology has a low sensitivity, ranging from 37% to 86%.38 In cubital tunnel syndrome, ulnar nerve CSA >10 mm2 is generally accepted as abnormal with sensitivity as high as 95% to 100%,39 but there is poor correlation between ulnar nerve size and electrophysiology. Ultrasound demonstrates a fusiform hypoechoic swelling of the ulnar nerve with loss of the fascicular pattern.40 Ultrasound examination during elbow flexion should always be obtained to identify dynamic nerve impingement (e.g., prominent medial head of triceps) (Fig. 12.9).
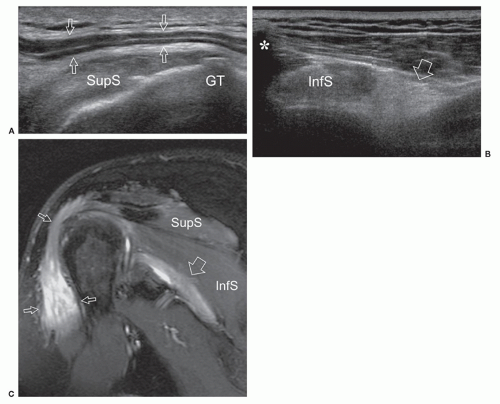
Anterior to the lateral epicondyle, the radial nerve gives off the posterior interosseous nerve that pierces supinator between the superficial and deep heads (radial tunnel) to reach the posterior compartment of the forearm. At the proximal edge of supinator, the nerve is bridged by a fibrous arch, the arcade of Fröhse, which joins the brachialis and brachioradialis muscles, the medial edge of the extensor carpi radialis brevis and the superficial belly of the supinator muscle. Ultrasound shows the posterior interosseous nerve as it pierces the supinator muscle. Posterior interosseous neuropathy may be caused by nerve compression at the arcade of Fröhse as a result of fibrous bands, fan-shaped recurrent radial vessels (leash of Henry), or tightness of the passage within the supinator. The main ultrasound patterns include impingement
of the nerve against the fibrous arcade or fusiform nerve swelling in the middle of the tunnel41,42 (Fig. 12.10). This latter location of nerve abnormality may be related to either compression (fibrous bands) or stretching trauma.
of the nerve against the fibrous arcade or fusiform nerve swelling in the middle of the tunnel41,42 (Fig. 12.10). This latter location of nerve abnormality may be related to either compression (fibrous bands) or stretching trauma.
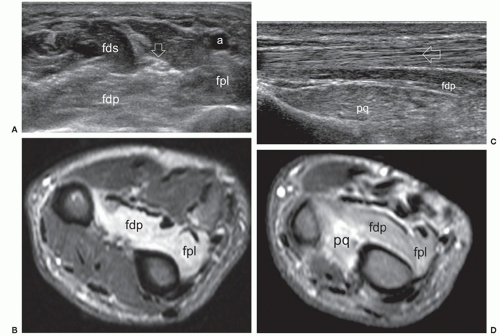
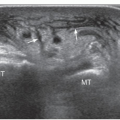
The anterior interosseous nerve is a motor branch of the median nerve that descends the forearm along the anterior surface of the interosseous membrane to supply the flexor pollicis longus, part of the flexor digitorum profundus (for the index and middle finger), and the pronator quadratus. Anterior interosseous neuropathy (Kiloh-Nevin syndrome) presents with an impaired pinch grip and may occur either above the elbow, when the nerve is still part of the median nerve, by a supracondylar bony spur and ligament of Struthers, or in the forearm at the point where the median and anterior interosseous nerves pass deep to the tendinous bridge connecting the humeroulnar and radial heads of the flexor digitorum superficialis muscle. The anterior interosseous nerve may be compressed by fibrous bands arising from the pronator teres or the flexor digitorum superficialis and anomalous muscles in the forearm (i.e., Gantzer muscle). In general, ultrasound examination of the anterior interosseous nerve is inconclusive in the absence of a mass because it is so small and deep. Besides direct nerve assessment, ultrasound may suggest the diagnosis due to loss of bulk and the hyperechoic pattern of the innervated muscles (Fig. 12.11).43,44
Tip:
Entrapment neuropathies are characterized by focal nerve enlargement at or near the site of compression, typically recorded as an increase in CSA.
Ultrasound can identify a wide spectrum of causes of entrapment neuropathy in the upper extremity.
The nerves of the upper extremity may be affected by compression or entrapment at specific anatomic locations (i.e., paraglenoid for the suprascapular nerve, quadrilateral space for the axillary nerve, cubital and Guyon tunnels for the ulnar nerve, and the supinator area for the posterior interosseous nerve).
Wrist
Carpal tunnel syndrome (CTS) is common (estimated prevalence 50 per 1,000 individuals per year). There is no gold standard for the diagnosis of CTS. Many physicians rely on the clinical presentation of pain at the wrist radiating into thumb, index, and middle fingers, with numbness and tingling, nocturnal wakening, thenar atrophy, Tinel sign, and Phalen sign. Others rely on electrodiagnostic (EDX) testing, but nerve conduction studies and EMG have an estimated sensitivity ranging from 85% to 98%. Finally, some institutions consider
treatment response (i.e., carpal tunnel injection, splinting, and surgical release) as a diagnostic feature of value. With such uncertainty regarding the gold standard, ultrasound provides direct depiction of size and morphologic abnormalities of the median nerve45 and is able to rule out anatomic variants (e.g., bifid nerve and persistent median artery) and space-occupying masses (e.g., ganglion cysts, accessory muscles, and flexor tenosynovitis). Besides assessing nerve size, ultrasound shows echotextural abnormalities, volar bulging of the flexor retinaculum, flattening of the median nerve in the distal carpal tunnel, and reduced passive sliding of the nerve during flexor tendon gliding (Fig. 12.12). Although CSA is the most accurate and reliable measure of nerve size, there is no consensus on the optimal threshold value for diagnosing median nerve compression. Diagnostic cutoff values range from 9 to 13 mm2. Most studies choose >10 or 11 mm2 at the carpal tunnel inlet.46 Instead of absolute cutoff values, a wrist-to-forearm ratio of median nerve CSA has been recently introduced.47 The CSA is determined at the level of the distal wrist crease and 12 cm proximally in the forearm. An upper normal limit of wrist-to-forearm ratio of 1.4 has 100% sensitivity for detecting CTS with no false positives.47 Others have proposed the pronator quadratus muscle as the level for the second CSA measurement. A difference (ΔCSA) between the largest CSA of the median nerve at the level of the carpal tunnel and the proximal third of the pronator quadratus is then calculated.48 A ΔCSA threshold of 2 mm2 has sensitivity and specificity as high as 99% and 100%, respectively for CTS.48 The same method can be used by combining the CSAs of the radial and ulnar branches of a bifid median nerve.49 Comparison with the opposite side has been suggested as an alternative for objective assessment of nerve size.31 This seems particularly promising in mild initial entrapment, when electrophysiology
is positive but the CSA is within or at the limits of normal. A difference in the CSA between sides might increase the examiner’s confidence that mild compression exists.
treatment response (i.e., carpal tunnel injection, splinting, and surgical release) as a diagnostic feature of value. With such uncertainty regarding the gold standard, ultrasound provides direct depiction of size and morphologic abnormalities of the median nerve45 and is able to rule out anatomic variants (e.g., bifid nerve and persistent median artery) and space-occupying masses (e.g., ganglion cysts, accessory muscles, and flexor tenosynovitis). Besides assessing nerve size, ultrasound shows echotextural abnormalities, volar bulging of the flexor retinaculum, flattening of the median nerve in the distal carpal tunnel, and reduced passive sliding of the nerve during flexor tendon gliding (Fig. 12.12). Although CSA is the most accurate and reliable measure of nerve size, there is no consensus on the optimal threshold value for diagnosing median nerve compression. Diagnostic cutoff values range from 9 to 13 mm2. Most studies choose >10 or 11 mm2 at the carpal tunnel inlet.46 Instead of absolute cutoff values, a wrist-to-forearm ratio of median nerve CSA has been recently introduced.47 The CSA is determined at the level of the distal wrist crease and 12 cm proximally in the forearm. An upper normal limit of wrist-to-forearm ratio of 1.4 has 100% sensitivity for detecting CTS with no false positives.47 Others have proposed the pronator quadratus muscle as the level for the second CSA measurement. A difference (ΔCSA) between the largest CSA of the median nerve at the level of the carpal tunnel and the proximal third of the pronator quadratus is then calculated.48 A ΔCSA threshold of 2 mm2 has sensitivity and specificity as high as 99% and 100%, respectively for CTS.48 The same method can be used by combining the CSAs of the radial and ulnar branches of a bifid median nerve.49 Comparison with the opposite side has been suggested as an alternative for objective assessment of nerve size.31 This seems particularly promising in mild initial entrapment, when electrophysiology
is positive but the CSA is within or at the limits of normal. A difference in the CSA between sides might increase the examiner’s confidence that mild compression exists.
There is increasing interest in the potential value of ultrasound as an indicator of disease severity. Moderate correlations between CSA and electrophysiologic severity exist, but do not reach the level of clinical significance.50,51 The lack of a stronger correlation may be due to the duration of disease and patient age.52 Emerging roles for nerve ultrasound in CTS include prognosis, selection of treatment options, and monitoring response to treatments. For example, a low median nerve CSA is more likely to respond to steroid injection into the carpal tunnel than a very thick nerve.53 In patients with persistent symptoms after decompressive surgery, ultrasound can demonstrate incomplete resection of the flexor retinaculum, scar tissue around the nerve, or injury of the palmar cutaneous branch (Fig. 12.13).54,55 Much remains to be learned about nerve ultrasound in CTS, but ultrasound is already changing the approach to diagnosis and treatment, although its role has yet to be established.
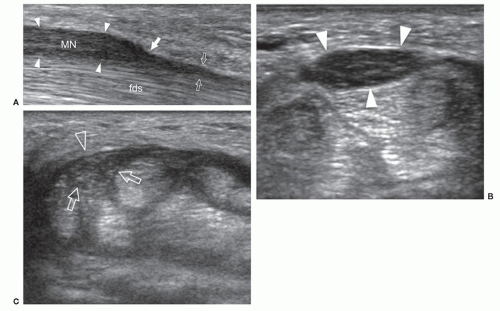
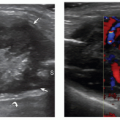
Guyon canal is a rare site of ulnar nerve entrapment (Fig. 12.14). Causes include space-occupying lesions such as pisotriquetral ganglia, ulnar artery pseudoaneurysms, and accessory muscles (i.e., AADM).21 Compression may involve the ulnar nerve prior to its bifurcation (zone-1) or be limited to one of its divisional branches, the superficial sensory (zone-2) or the deep motor (zone-3). The ulnar nerve is easily identified in Guyon canal between the pisiform and ulnar artery using short-axis planes. Moving the transducer distally, the superficial branch can be found superficial to the hamate hook, while the deep motor branch courses along the medial side of the hamate. External compression, either acute or repetitive (cyclist palsy), may cause nerve injury by squeezing it against the hamate hook. This type of injury may also cause occlusion of the ulnar artery (hypothenar hammer syndrome).
Tip:
In CTS, the CSA of the median nerve is increased at the proximal edge of the retinaculum and the nerve is flattened within the distal tunnel.
There is no consensus on what is an abnormal CSA measurement of the median nerve: proposed absolute threshold value is approximately 10 to 10.5 mm2.
To reduce the impact of intersubject and internerve variability, comparison between the CSA of the median nerve obtained at the carpal tunnel and more proximally seems a promising alterative.
Hip
The main entrapment neuropathies about the hip amenable to ultrasound examination involve the lateral femoral cutaneous nerve and the femoral nerve.
The lateral femoral cutaneous nerve is a purely sensory nerve arising from L2-L3. It descends in the pelvis alongside the iliopsoas and enters the thigh deep to the inguinal ligament or through a split in the ligament close to the anterior superior iliac spine. It then divides into terminal branches to the anterolateral thigh. Lateral femoral cutaneous neuropathy (meralgia paresthetica) is caused by entrapment where the nerve crosses the inguinal ligament, and consists of numbness and sensory disturbance in the anterolateral thigh. The main ultrasound signs are fusiform nerve swelling, usually proximal to the ligament (Fig. 12.15).56,57,58 Short-axis scans may help to show changes in nerve shape and size across the ligament.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree