Aorta
3.1.1 Anatomy
The aorta is the largest arterial vessel in the body. It is divided into several anatomical segments, each of which is subject to specific diseases. The frequency of congenital disorders in particular varies significantly in different aortic segments.
Histologically, the aorta is classified as an elastic artery. As for all vessels in the body, its wall is composed of three layers (from inside to outside):
Intima: The intima consists of endothelium and subendothelial connective tissue with delicate collagen fibers. Endothelial cells are biologically active and secrete vasodilators and vasoconstrictors as required. The aorta has a relatively thick intima, consistent with the mechanical stresses to which the vessel is exposed.
Media: The media consists of concentric layers of smooth muscle cells, collagen fibers, and elastic fibers. The latter form an internal elastic membrane between the intima and media and an external elastic membrane between the media and adventitia. The media absorbs vascular tension and regulates the vascular caliber.
Adventitia: The adventitia surrounds the media with a stabilizing meshwork of collagen fibers and elastic lamellae. The adventitia contains the vasa vasorum, which extend into the outer third of the media and supply blood to the aortic wall.
The principal anatomical segments of the aorta are the aortic root, the ascending aorta, the aortic arch, the descending thoracic aorta, and the abdominal aorta (▶ Fig. 3.1).

Fig. 3.1 Anatomy of the aorta. Diagrammatic representation of the ascending aorta, aortic arch, descending aorta, abdominal aorta, and the major supra-aortic and abdominal branch vessels. (Reproduced from Schünke M, Schulte E, Schumacher U. Prometheus. LernAtlas der Anatomie: Innere Organe. Illustrated by M. Voll/K. Wesker. 2nd ed. Stuttgart: Thieme; 2009.)
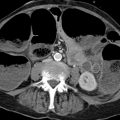
Aortic root: The aortic root is the short segment from the aortic valve to the sinotubular junction. The left and right coronary arteries arise from the aortic root.
Ascending aorta: The segment just above the aortic root is the ascending aorta, which extends to the origin of the first supra-aortic branch vessel, the brachiocephalic trunk. The average ascending aorta is approximately 5 cm long and 3.5 cm in diameter.
Aortic arch: The aortic arch extends to the ductus arteriosus or ligamentum arteriosum. 1 It gives off the supra-aortic branch vessels, which are, from proximal to distal, the brachiocephalic trunk, left common carotid artery, and left subclavian artery. Approximately 2 cm from its origin, the brachiocephalic trunk divides into the right subclavian artery and right common carotid artery. The distal segment of the aortic arch from the distal circumference of the left subclavian artery to the ductus arteriosus or ligamentum arteriosum is called the aortic isthmus. The aortic arch is typically located to the left of the midline. It is classified into three types based on the relative position of the brachiocephalic trunk (▶ Fig. 3.2): With a type I aortic arch, the origin of the brachiocephalic trunk is level with the outer curvature (top) of the arch. With a type II aortic arch, the origin of the brachiocephalic trunk is at a level between the outer and inner curvatures of the arch. With a type III arch, the origin is below the level of the inner curvature. This distinction is particularly important for planning interventional therapies, as the interventional approach to the supra-aortic vessels becomes more difficult with increasing complexity of aortic arch anatomy (types II and III), with a corresponding rise in complication rates.
Descending thoracic aorta: The next segment is the descending thoracic aorta, which continues to the diaphragm. The proximal part of this segment, just distal to the ductus arteriosus, may show a slight physiologic expansion. The descending thoracic aorta gives off the segmentally arranged intercostal arteries and the bronchial arteries, which consistently arise at the level of the tracheal bifurcation. Other small-caliber branches are the pericardial, esophageal, and superior phrenic arteries.
Abdominal aorta: The infradiaphragmatic abdominal aorta extends from the diaphragm to the aortic bifurcation at the level of the T4 vertebra, where it divides into the common iliac arteries. The abdominal aorta gives off numerous large vessels to all abdominal structures. The most proximal branch vessel is the celiac trunk, which divides at once into the common hepatic artery, splenic artery, and left gastric artery. Just distal to the celiac trunk (approximately one-half vertebral body height) is the origin of the superior mesenteric artery, which supplies large portions of the small intestine, ascending colon, and proximal two-thirds of the transverse colon. The third unpaired branch vessel is the inferior mesenteric artery, which arises just above the aortic bifurcation and supplies the peripheral colon segments including the sigmoid colon and rectum. These vessel origins are subject to numerous variations, which should be noted in the planning of surgical and interventional procedures. The sacral artery branches from the posterior side of the abdominal aorta to supply the sacrum and coccyx. The largest paired branch vessels are the renal arteries. Proximal to them are the inferior phrenic arteries, which may also arise from one or both sides of the celiac trunk, and small suprarenal arteries, which often arise from the proximal part of the renal arteries. Paired lumbar arteries arise at segmental levels from the back of the aorta to supply the vertebral bodies, back muscles, and spinal cord. The different vascular territories are interconnected by numerous collateral vessels, enabling them to divert blood to other territories that have lost their normal supply. The most familiar of these collaterals is the Riolan anastomosis, which creates a relatively large connection between the territories of the superior and inferior mesenteric arteries in the left upper abdomen.

Fig. 3.2 Classification of the aortic arch. Schematic representation. Classification is based on the relationship of the origin of the brachiocephalic trunk to the inner and outer curvatures of the aortic arch.
3.1.2 Development
The development of the aorta and its large branch vessels is a complex, synchronized process. A knowledge of this process is helpful for understanding congenital anomalies and variants. Ontogenetically, the arterial limb of the embryonic circulation begins with the still-undivided truncus arteriosus. This structure is connected with the aortic arch, which carries blood around the gut tube to the paired dorsal aortae. Starting in approximately the third week of gestation, a relatively small right and left ventral aorta is formed along with a larger right and left dorsal aorta. Development of the six branchial arches is paralleled by the development of six aortic arches, which interconnect the ventral and dorsal aortae on each side. The six aortic arches do not all coexist at any one time, however.
The first three aortic arches give rise to the arteries supplying the head and neck region. The first two aortic arches regress while the third one, along with parts of the dorsal aortae, form the major portion of the internal carotid arteries. The latter develop from the ventral aortae. The fourth aortic arch develops asymmetrically, in accordance with the asymmetric development of the heart. The left fourth aortic arch in the embryo persists as the aortic arch, while the right arch forms the brachiocephalic trunk and proximal right subclavian artery. The fifth aortic arch is absent or does not form definitive structures. The sixth aortic arches give rise to the proximal part of the pulmonary trunk, the left arch also forming the ductus arteriosus. Caudal to approximately the T4 vertebral level, the dorsal aortae fuse to form the definitive descending aorta (▶ Fig. 3.3). The originally paired yolk sac arteries fuse during embryonic development and form the unpaired visceral vessels in adults: the celiac trunk and the superior and inferior mesenteric arteries.

Fig. 3.3 Normal embryological development of the six aortic arches (I–VI). Schematic representation of the results of normal development. Portions of the dorsal aortae that disappear in approximately the seventh week of gestation are shown in gray. (Reproduced from Schünke M, Schulte E, Schumacher U. Prometheus. LernAtlas der Anatomie: Allgemeine Anatomie und Bewegungssystem. Illustrated by M. Voll/K. Wesker. 3rd ed. Stuttgart: Thieme; 2011.)
3.1.3 Congenital Anomalies
Approximately 20% of all cardiovascular malformations are aortic anomalies. 2 The proximal portions (closest to the heart) are most commonly affected because they undergo the most complex embryological development. The clinical presentation of aortic anomalies is extremely variable and ranges from asymptomatic to life-threatening. The most common anomalies affecting the thoracic aorta distal to the sinotubular junction are as follows:
Variants in the origins of supra-aortic vessels.
Aberrant right subclavian artery and Kommerell diverticulum.
Patent ductus arteriosus.
Coarctation of the aorta.
Double aortic arch.
Right aortic arch.
Variants in the Origins of Supra-aortic Vessels
The normal anatomy described above, with three supra-aortic branch vessels, is present in approximately 70% of the population. The actual arrangement can vary over a very broad range that includes ethnic differences. Possible variants in the origins of the supra-aortic vessels are listed in ▶ Table 3.1. The most frequent variant is a common origin of the brachiocephalic trunk and left common carotid artery (▶ Fig. 3.4). Another common variant is the left common carotid artery arising from the brachiocephalic trunk (▶ Fig. 3.5). Often this is erroneously called a “bovine arch”; by definition, the bovine aorta requires a single origin of all supra-aortic vessels from the aortic arch, a pattern consistently found in cattle. 3 Another variant is illustrated in ▶ Fig. 3.6.
Variant | Approximate frequency (%) |
Common origin of the brachiocephalic trunk and left common carotid artery | 13 |
Left common carotid artery arising from the brachiocephalic trunk | 9 |
Left vertebral artery arising directly from the aortic arch | 5 |
Left common carotid artery and left subclavian artery arising by a common trunk | 1 |
Aberrant origin of the right subclavian artery from the left side of the aortic arch | 0.5 |

Fig. 3.4 The most frequent variant in the origin of the supra-aortic vessels. Common origin of the carotid arteries from the aortic arch. (a) DSA. (b) CT after implantation of a thoracic stent.

Fig. 3.5 Variant in the origin of supra-aortic vessels. The left common carotid artery (arrow) arises from the brachiocephalic trunk (asterisk).

Fig. 3.6 Variant in the origin of supra-aortic vessels. The left vertebral artery arises directly from the aortic arch (arrow).
Aberrant Right Subclavian Artery and Kommerell Diverticulum
Brief definition An aberrant origin and course of the right subclavian artery is a clinically significant variant. Also known as a Kommerell diverticulum, it occurs when the right subclavian artery arises from the left side of the descending aorta rather than from the brachiocephalic trunk. Typically the aberrant vessel runs to the right and passes behind the esophagus (▶ Fig. 3.7). Less commonly it may run anterior to the esophagus (15% of cases) or, more rarely, anterior to the trachea (5% of cases). In this variant the recurrent laryngeal nerve does not form a loop around the right subclavian artery but runs directly to the laryngeal muscles. When the aberrant vessel passes behind the esophagus, it may cause “dysphagia lusoria” due to compression of the esophagus. Only about 10% of patients with a retroesophageal right subclavian artery become symptomatic, however, and the aberrant vessel is usually detected incidentally. Dysphagia lusoria is generally treated surgically.

Fig. 3.7 Kommerell diverticulum. Diagrammatic posterior view illustrates a Kommerell diverticulum as described by Burckhard in the original 1936 publication.
Imaging signs The chest radiograph in patients with an aberrant right subclavian artery may show widening of the superior mediastinum. This is caused by an outpouching of the aorta at the origin of the aberrant vessel. Occasionally the course of an aberrant right subclavian artery is identified on the chest film by vascular calcifications (▶ Fig. 3.8a). 4 A barium esophagogram may show an impression on the posterior esophageal wall. Sectional imaging studies, especially CT angiography (CTA) and MRI, can supply the most definitive results (▶ Fig. 3.8b).

Fig. 3.8 Examples of an aberrant right subclavian artery. (a) The chest radiograph shows widening of the superior mediastinum. In rare cases the course of the vessel is defined by the presence of vascular calcifications (arrows). (b) CT demonstrates the aberrant origin of the right subclavian artery. It arises from the aortic arch as the last supra-aortic vessel and usually runs behind the esophagus (asterisk) to the right side (arrows).
Patent Ductus Arteriosus
Brief definition The ductus arteriosus is the part of the fetal circulation that connects the aorta with the pulmonary arterial system. The ductus arteriosus runs from the anteromedial circumference of the aortic isthmus to the proximal left pulmonary artery. The ductus arteriosus normally undergoes functional closure within 24 hours after birth and anatomical closure in subsequent days and weeks, leaving behind only a bandlike structure, the ligamentum arteriosum. Patent ductus arteriosus is diagnosed if the ductus is still patent 3 months after birth. This entity is relatively common, with an incidence of 1:2,000 live births. It is particularly common in premature infants.
Imaging signs Echocardiography is the imaging study of choice for the diagnosis of a patent ductus arteriosus. Depending on the shunt volume, the plain chest film may show indirect signs such as an enlarged cardiac silhouette or dilated pulmonary vessels indicative of pulmonary venous hyperemia or pulmonary edema. CT and MRI are excellent for demonstrating the presence of a patent ductus and classifying its morphology. 5
Clinical features A continuous “machinery murmur” is heard on auscultation. The symptoms depend on the magnitude of the shunt volume and the presence of associated cardiac anomalies. An enlarged left ventricle and pulmonary hypertension are typical manifestations of a patent ductus arteriosus. With some congenital anomalies such as pulmonary atresia, a patent ductus arteriosus is necessary so that lung perfusion, for example, can be maintained by flow across the shunt. Treatment options depend on the timing of diagnosis and the morphology of the patent ductus; they include pharmacologic therapy (indomethacin) as well as surgical and interventional options.
Differential diagnosis Even when the ductus arteriosus closes normally, a localized anteromedial outpouching may be found at the position of the ductus. This variant, called a “ductus bump,” is found in up to one-third of newborns and approximately 9% of adults. It can be distinguished clinically from a traumatic aortic rupture, which occurs most commonly at the site of the ligamentum arteriosum.
Key points A patent ductus arteriosus connects the aorta with the pulmonary arterial circulation, running from the aortic isthmus to the left pulmonary artery. It is diagnosed by echocardiography. The chest radiograph shows signs of a shunt defect with an increased volume load on the cardiopulmonary system. Sectional imaging by MRI or CTA allows direct visualization of the patent ductus for planning interventional therapy, etc.
Coarctation of the Aorta
Brief definition Coarctation of the aorta is a congenital narrowing of the thoracic aorta at the level of the aortic isthmus. A preductal (infantile) form is distinguished from a postductal (adult) form based on the relationship of the lesion to the ductus arteriosus. These forms also have different clinical manifestations. Coarctation of the aorta occurs in approximately 0.3% of all live births and comprises 5 to 8% of all congenital cardiac anomalies. It is associated with various syndromic conditions such as Turner’s syndrome (20% of cases) and other cardiac anomalies such as a bicuspid aortic valve.
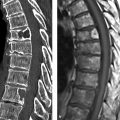
Imaging signs The infantile form of coarctation usually produces nonspecific radiographic changes such as cardiac enlargement and signs of pulmonary venous congestion. A possible direct sign on chest films is the epsilon or figure-3 sign, which reflects the atypical shape of the narrowed aorta. In the adult form, a longstanding coarctation may cause rib notching from enlarged intercostal vessels functioning as collaterals (▶ Fig. 3.9). Notching most commonly affects the fourth through eighth ribs. The first two ribs are always spared, as they are supplied by the costocervical trunk. Direct detection of the lesion, including visualization of the collateral pathways, can be accomplished with CTA (▶ Fig. 3.10) or MRI (▶ Fig. 3.11). MRI also permits flow measurements for assessing hemodynamic significance.

Fig. 3.9 Adult form of coarctation of the aorta. The chest radiograph displays the typical rib notches, seen here predominantly in the fourth through seventh ribs (arrowheads in magnified view).

Fig. 3.10 Adult form of coarctation of the aorta. CTA. (a) Sagittal reformatted CTA image shows the stenosis (arrow) distal to the origin of the left subclavian artery (arrowheads), which is greatly dilated due to massive collateral flow (asterisk). (b) Axial reformatted CTA image displays the numerous collateral vessels.

Fig. 3.11 Infantile form of coarctation of the aorta. MRA. In this case the stenosis (arrow) is proximal to the origin of the left subclavian artery (arrowheads) and thus proximal to the ductus arteriosus. This is a moderate coarctation, as indicated by the absence of prominent collaterals.
Note
When the dimensions of a coarctation are determined at sectional imaging, it is important to obtain a true cross section for each vascular segment and avoid oblique sections.
Clinical features The clinical presentation depends on the type of aortic coarctation, i.e., whether it is a preductal (infantile) form or a postductal (adult) form:
Infantile form: In this form the stenosis is located proximal to the ductus arteriosus, and the heart pumps against the resistance of the stenosis. Because this proximal type of coarctation is poorly collateralized, patients consistently develop left-sided heart failure and pulmonary venous congestion with dyspnea. The ductus arteriosus typically remains patent, causing venous blood to be shunted to the lower half of the body with resulting cyanosis of the trunk and lower limbs. The poor oxygenation may lead to organ failure.
Adult form: The adult, postductal form of coarctation allows for the compensation of arterial blood flow by collateral vessels. The ductus arteriosus obliterates normally, and cyanosis does not occur; however, physical examination typically reveals hypertension in the upper half of the body where the heart must pump against a resistance. This may lead to a pulse and blood pressure discrepancy between the upper and lower halves of the body.
The infantile form has a mortality rate up to 90% in untreated cases. Treatment is urgent, therefore, and usually consists of primary surgery. The adult form should also be corrected in childhood but is an elective indication. Older children or patients with restenosis should be treated by stent implantation or repeated balloon dilatation.
Differential diagnosis The adult form of aortic coarctation must be differentiated from pseudocoarctation due to atypical elongation and kinking of the aorta and from large-vessel arteritis such as Takayasu’s arteritis.
Key points Preductal and postductal forms of aortic coarctation are distinguished according to their relationship to the ductus arteriosus as seen on imaging. While the chest radiograph may show specific signs such as the epsilon sign and rib notching, direct visualization by MRI or CTA is state-of-the-art. Assignment of cases to the adult or infantile form is important for directing further treatment and is an essential part of the radiology report.
Double Aortic Arch
Brief definition Double aortic arch is part of a complex group of congenital anomalies that can be collectively referred to as aortic vascular rings:
Double aortic arch.
Right aortic arch:
With an aberrant left subclavian artery and left ligamentum arteriosum.
With mirror-image branching and a retroesophageal ligamentum arteriosum.
Left aortic arch:
• With an aberrant right subclavian artery and right ligamentum arteriosum.
• With a right descending aorta and ligamentum arteriosum.
These anomalies are well explained by the hypothetical model of the double aortic arch postulated by J. E. Edwards in the early 1950s (▶ Fig. 3.12). 6 In this model, the various possible forms of vascular rings can be traced to different problems in the development and regression of specific structures included in the model. Double aortic arch is the most common vascular ring anomaly, accounting for up to 60% of cases in this group (▶ Fig. 3.13). Embryologically, this variant results from bilateral persistence of the fourth aortic arches. Morphologically, the right aortic arch is the dominant arch in approximately 70% of cases. The left arch is dominant in approximately 25% of cases. Occasionally, both arches are of equal size. With partial atresia of one of the aortic arches, the vascular ring may be completed by fibrotic residues of the atretic vascular segment. This variant of the aortic arch anomaly is rarely associated with congenital heart disease.

Fig. 3.12 Double aortic arch. Hypothetical model of Edwards. The letters A–D designate potential regression sites for various parts of the aortic arches.

Fig. 3.13 Aortic ring anomaly with a double aortic arch. Two patients. (a) Axial CT demonstrates a dominant right aortic arch (black asterisk) and a smaller left aortic arch (arrows). Thymic tissue (white asterisk) is visible in the anterior mediastinum. (b) 3D reconstruction of CT data from a different patient shows the four-vessel sign with a subclavian artery and common carotid artery arising from each of the two aortic arches. The left arch is dominant in this patient. A nasogastric tube (white) marks the location of the esophagus.
Imaging signs The barium esophagogram may show posterior indentation of the trachea or esophagus, depending on the cause of the vascular ring. The sensitivity and specificity of this technique are relatively poor, however. Contrast-enhanced low-dose CT is the modality of choice for initial imaging, while MRI should be used for follow-up. If the vascular ring does not lie in one axial plane of section, then the “four-vessel sign” with the right subclavian artery and right common carotid artery arising from the right arch and the left common carotid and subclavian arteries arising from the left arch will help to identify this anomaly.
Clinical features Patients present clinically with dysphagia or respiratory problems. Respiratory complaints such as stridor are more common in newborns and children, while dysphagia is dominant in older children and undiagnosed adults. Treatment involves surgical division of the smaller aortic arch and ligamentum arteriosum.
Right Aortic Arch
Brief definition A right aortic arch results from involution of the fourth left aortic arch and is found in approximately 0.1% of adults. This variant may occur alone or may be associated with cyanotic congenital heart disease, especially the tetralogy of Fallot. Different types of right aortic arch are distinguished according to the origin of the supra-aortic vessels, and certain configurations may produce a vascular ring (see above).
The most common form, accounting for 59 to 84% of cases, is a right aortic arch with mirror-image branching relative to normal anatomy.
The second most common form (14–39% of cases) is a right aortic arch with an aberrant left subclavian artery. This artery is the last branch vessel from the aortic arch and often shows proximal dilatation analogous to a Kommerell diverticulum.
A very rare form (less than 1% of cases) is a right aortic arch with an isolated left subclavian artery, which may lead to a congenital subclavian steal syndrome and vertebrobasilar insufficiency.
Caution
When describing the finding of a right aortic arch, it is important to be aware that the terminology used in the literature is not uniform, and it is best to provide a verbal description as illustrated above.
Imaging signs The left aortic border is absent in the chest radiograph. The right aortic arch typically appears at a high right paratracheal site, while the aortic border occupies a right paravertebral position (▶ Fig. 3.14). Tracheal indentation may be visible at the level of the aortic arch. Symptomatic patients require sectional imaging evaluation for detection and classification of a vascular ring, for example.

Fig. 3.14 Right aortic arch and a right descending aorta in a 47-year-old woman. The right descending aorta is clearly visualized in its right paravertebral location. The trachea and esophagus are displaced to the left by the right aortic arch. The esophagus is identified by a nasogastric tube.
Congenital Abdominal Aortic Stenosis
Clinical features The symptoms of congenital abdominal aortic stenosis are similar to those of the adult form of coarctation of the aorta, with arterial hypertension and a blood-pressure discrepancy between the upper and lower limbs. Blood flow distal to the stenosis may be significantly decreased, leading to peripheral claudication or even renal failure. Treatment is usually surgical. The role of interventional treatments is still greatly limited as a result of numerous setbacks. 7, 8
Brief definition Midaortic syndrome is a variant of congenital aortic stenosis (▶ Table 3.2). Although approximately 98% of cases present as coarctation of the aorta, the stenosis may be located farther distally in 0.5 to 2% of cases and is then termed “midaortic syndrome.” Many other names have also been applied to this condition such as “hypoplasia of the abdominal aorta,” “congenital abdominal aortic stenosis,” and “coarctation of the abdominal aorta.” Midaortic syndrome may occur in the descending thoracic aorta or in the abdominal aorta. The site of occurrence is highly variable and has also been described as suprarenal or infrarenal.
Ascending thoracic aorta | Aortic arch | Descending aorta |
|
|
|
3.1.4 Diseases
Aortic Aneurysms
Brief definition An aneurysm is defined as the permanent dilatation of a vessel by at least 50% of its normal diameter. 9 Given that the diameter of the aorta, especially its thoracic portion, increases with aging, it is reasonable to assume that the midportion diameter of the ascending aorta should not exceed 4 cm while the diameter of the descending aorta should not exceed 3 cm.
A basic distinction is made between true and false aneurysms. A true aneurysm involves all three layers of the vessel wall (intima, media, adventitia) while a false aneurysm, also called a pseudoaneurysm, does not. Many pseudoaneurysms involve only the adventitia and periadventitial tissue. Typical causes of pseudoaneurysms include aortic dissection and posttraumatic aneurysms. The causes of true aortic aneurysms are extremely diverse, the most common being degenerative changes in the aortic wall due to atherosclerosis. This mechanism is responsible for 30% to more than 80% of aortic aneurysms in different studies. Other relatively frequent causes of aortic aneurysms are aortic dissection, inflammatory changes, and congenital disorders (▶ Table 3.3), most notably Marfan’s syndrome and Ehlers–Danlos syndrome. Marfan’s syndrome in particular leads to early, significant aneurysmal growth.
Cause | Frequency (%) |
Common | |
Atherosclerosis | ca. 70 (30–80) |
Aortic dissection | <50 |
Aortitis (infectious) | 12 |
Media degeneration | 6 |
Marfan’s syndrome | 5–10 |
Rare | |
Ehlers–Danlos syndrome | |
Turner’s syndrome | |
Bicuspid aortic valve | |
Trauma | |
Noninfectious aortitis:
| |
Scleroderma | |
Osteogenesis imperfecta | |
Radiation-induced | |
Imaging signs Asymptomatic aortic aneurysms, unlike symptomatic lesions, are generally detected incidentally in imaging studies ordered for a different indication. Aortic aneurysms most commonly involve the abdominal aorta (31%), followed by the ascending aorta (22%), the aortic arch (11%), and the descending aorta (7.5%). Thoracoabdominal aortic aneurysms are relatively rare, comprising approximately 3% of cases.
Caution
The diagnostic work-up of an aneurysm should include imaging of the entire aorta because, for example, 28% of patients diagnosed with a thoracic aortic aneurysm are also found to have an abdominal aneurysm (▶ Fig. 3.15).
The chest radiograph shows widening of the aortic contour. Calcifications in the aortic wall can be very helpful in determining vessel diameter and are often seen most clearly in the lateral chest film. In extreme cases an aneurysm of the ascending aorta may occupy all of the retrosternal space (▶ Fig. 3.16). The abdominal plain film can clearly demonstrate the presence and size of an aneurysm when conspicuous calcifications are present, but the sensitivity of this technique is very low. In some cases an abdominal aortic aneurysm is also indicated by its displacement of intestinal structures. Ultrasound, CT, and MRI can generally provide a definitive diagnosis by showing aortic enlargement to more than 4 cm in the ascending aorta and more than 3 cm in the descending and abdominal aorta.

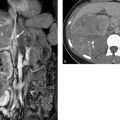
Fig. 3.15 Large thoracic abdominal aneurysm (Estrera type A).10 (a) PA chest radiograph shows a very large mass in the left apical region. The lesion is distinguishable from a malignant tumor by its relationship to the aorta, its pushing margins, and the absence of local destructive changes in the chest wall, for example. (b) Lateral chest radiograph. (c) CTA accurately localizes the aneurysm and also demonstrates a mural thrombus. (d) The entire aorta should be imaged at initial work-up, as one-third of patients with a thoracic aortic aneurysm have additional aneurysms. In the case shown, the thoracic aneurysm (arrow) coexists with other aneurysms of the right common iliac artery and internal iliac artery (arrowheads).

Fig. 3.16 Aneurysm of the ascending aorta. Chest radiographs (a, b) show widening of the aortic shadow (double-headed arrows) with the aortic contour normalizing toward the aortic arch. Axial CT scan at right angles to the vessel (c) permits an accurate measurement of the vascular diameter (e.g., for preoperative planning). (a) PA chest radiograph. (b) Lateral chest radiograph. (c) CT, axial.
Note
Especially in patients with a presumed aneurysm of the ascending aorta, ECG-gated imaging should be done to eliminate motion artifacts. The aortic valve should also be evaluated because of the strong association of ascending aortic aneurysm with a bicuspid aortic valve.
Besides making the diagnosis, important imaging goals with an aortic aneurysm are the determination of its size, follow-up, and treatment planning. Another purpose in high-risk groups is screening for the presence of an aortic aneurysm. While this should rely mainly on ultrasound, CTA has become the current clinical standard for detecting aneurysm extension and planning treatment. The follow-up of abdominal aneurysms can be done with ultrasound in patients under age 65 years; CT is the accepted clinical standard in older patients. In comparing imaging measurements, it is important to note that ultrasound underestimates vascular diameters by approximately 4 mm compared with CT. MRI is also effective for detecting aneurysm extension but is less widely used for this indication due to factors such as high cost and limited availability. Aortic aneurysms are classified on the basis of sectional imaging findings. Classification is useful for directing treatment and predicting outcomes. The Estrera classification is used for thoracic aneurysms (▶ Fig. 3.17), 10 the Crawford classification for thoracoabdominal aortic aneurysms (▶ Fig. 3.18, ▶ Fig. 3.19). 11

Fig. 3.17 Estrera classification of thoracic aortic aneurysms. 10 The Estrera classification identifies three types of aneurysm as involving the upper or lower aorta (proximal or distal to the sixth intercostal artery) or the entire thoracic aorta. Types A and B aneurysms have a much lower risk of spinal complications than type C in surgical and interventional procedures. 10

Fig. 3.18 Crawford classification of thoracoabdominal aortic aneurysms. Type 1 extends from the left subclavian artery to a level above the renal arteries and type 2 from the left subclavian artery to a level below the renal arteries. Type 3 extends from below the sixth intercostal artery to a level below the renal arteries, type 4 from below the 12th intercostal artery to a level below the renal arteries. Type 5 was added later to denote an aneurysm starting below the sixth intercostal artery and extending to a level above the renal arteries.

Fig. 3.19 Thoracoabdominal aortic aneurysm (Crawford type 4). (a) 3D reconstruction displays the infrarenal extent of the aneurysm. (b) Axial CT scans demonstrate involvement of the visceral vascular segment. (c) 3D reconstruction after interventional therapy with a quadruple fenestrated aortic stent. At this point the aneurysm is excluded from the circulation and the visceral vessels are well perfused. (d) Axial CT scan after interventional therapy. The aneurysm sac is completely thrombosed.
Note
It is important to follow a uniform measurement standard for aortic aneurysms to ensure accurate follow-up. The measurement of aortic diameter is based on well-defined reference points in a true short-axis view of the vessel (see ▶ Fig. 3.16).
Use of the following reference points is recommended:
Sinotubular junction.
Proximal aortic arch.
Proximal descending aorta.
Aorta at the level of the diaphragm.
Infrarenal aorta (approximately 2 cm below the renal arteries).
Aortic bifurcation.
Maximum sectional diameter of any aortic aneurysms that are present.
The recommended intervals for follow-up imaging are based on the size of the aneurysm (▶ Table 3.4). It is also important to note typical postoperative changes such as elephant trunk, graft kinking, and foreign materials as well as typical complications such as perigraft hematoma and abscess, fistulas, and pseudoaneurysms. 12, 13
Maximum aneurysm size (cm) | Follow-up interval |
<3.0 | None |
3.0–4.0 | Once a year |
4.0–4.9 | Every 6 months |
5.0 or larger | Initiate treatment |
Clinical features Most aortic aneurysms are clinically silent and are detected incidentally. Symptomatic aneurysms usually present with thoracic, abdominal, or back pain, depending on their location, or become symptomatic only when they rupture. In rare cases thrombotic material from the aneurysm wall may cause a peripheral embolism with associated symptoms. The complication rate correlates with aneurysm size (▶ Table 3.5). Moreover, thoracic aortic aneurysms in particular may cause compression-related symptoms such as hoarseness due to compression of the recurrent laryngeal nerve, stridor or dyspnea due to airway compression, and a superior or inferior vena cava syndrome.
Risk (%/year) | Complication rate (%) | ||
Diameter >4 cm | Diameter >5 cm | Diameter >6 cm | |
Rupture | 0.3 | 1.7 | 3.6 |
Dissection | 1.5 | 2.5 | 3.7 |
Death | 4.6 | 4.8 | 10.8 |
At least 1 complication | 5.3 | 6.5 | 14.1 |
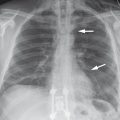
Differential diagnosis Elongation of the aorta and aortic dissection are the most common differential diagnoses on radiographs. Motion artifacts in the ascending aorta can mimic aortic dissection on non-ECG-gated CT. Rarely, tumors or congenital anomalies such as a Kommerell diverticulum can mimic an aneurysm. Similarly, the left superior intercostal vein may simulate an outpouching of the aortic arch (“aortic nipple”; ▶ Fig. 3.20). This variant may be found in approximately 5% of the population. The differential diagnosis on sectional imaging includes a penetrating aortic ulcer and ductus diverticulum.

Fig. 3.20 Aortic nipple. (a) The aortic nipple (arrows), visible on the PA chest radiograph, should be included in the differential diagnosis of thoracic aortic aneurysms and penetrating aortic ulcer. (b) CT identifies the feature as a projection effect caused by the left superior intercostal vein.
Key points A distinction is made between true and false aortic aneurysms. Diagnostic imaging should cover the entire aorta, at least initially, because aneurysms are often multifocal. Both CTA and MR angiography (MRA) are suitable as diagnostic modalities. An aneurysm is present if the midportion diameter of the ascending aorta is greater than 4 cm or the diameter of the descending or abdominal aorta is greater than 3 cm. Follow-up should employ standardized measurements and is important because the risk of aneurysm rupture rises sharply with increasing absolute diameter and rate of enlargement.
Subgroup: Mycotic Aneurysms
Brief definition Mycotic aneurysms are a subgroup of aortic aneurysm caused by bacterial infection of the vessel wall. The name, which erroneously implies a fungal infection, refers to the typical “mushroom” shape of mycotic aneurysms. Risk factors include atherosclerosis, arterial grafts and catheters, joint replacement, alcoholism, IV drug abuse, steroid therapy, chemotherapy, and diabetes mellitus. Mycotic aneurysms account for 0.5 to 3.0% of all aortic aneurysms. The aorta is most commonly affected, followed by the femoral artery and brachial artery. Mycotic aneurysms are rare in the intracerebral and mesenteric vessels, but essentially any vessel may be affected.
Imaging signs Mycotic aneurysms usually show a mixed pattern of fusiform and saccular aortic dilatation with regular wall thickening. Rarely, intramural or perianeurysmal gas inclusions may be detected. Surrounding fat imbibition and fluid inclusions may occur. An enhancing periaortic soft tissue component may be present. Sites of vertebral body erosion, abscesses (especially psoas abscess), or embolic organ infarction may occur, depending on the location of the aneurysm. Mycotic aneurysms are relatively dynamic and may undergo rapid changes in their shape and size.
Clinical features Nonspecific symptoms such as fever and local inflammatory signs may be suggestive in patients with peripheral mycotic aneurysms. Abdominal, back or flank pain may occur, depending on the site of occurrence. Paresthesias or neurologic symptoms have also been described. Approximately 7% of all mycotic aneurysms are asymptomatic. Mycotic aneurysms of peripheral arteries are associated with a palpable, pulsating mass. Untreated cases have a poor prognosis, with an approximately 50% rate of spontaneous rupture or uncontrolled septic complications. Even with treatment, mycotic aortic aneurysms have a reported mortality rate as high as 75%. Peripheral aneurysms have a single-digit mortality rate, with an amputation rate as high as 20%.
Differential diagnosis The differential diagnosis includes all other forms of spontaneous aortic aneurysm, penetrating aortic ulcer, and Kommerell diverticulum.
Key points Mycotic aneurysms result from bacterial inflammation and have a very poor prognosis without treatment. At imaging, the presence of a lobulated or mixed fusiform-saccular aneurysm with associated periaortic fluid or soft tissue component should suggest the correct diagnosis.
Acute Aortic Syndrome
Brief definition “Acute aortic syndrome” is a collective term for a group of etiologically diverse diseases of the thoracic aorta, aortic dissection, intramural hematoma, and penetrating aortic ulcer. The incidence is approximately 3:100,000 population per year.
Imaging signs The basic imaging tool is the plain chest radiograph. Possible findings are mediastinal widening, opacity of the aortopulmonary window, widening of the mediastinal silhouette, and displaced intimal calcifications, especially with an aortic dissection. More than 20% of patients have a normal chest film, however, and symptomatic cases will require further investigation. With the high spatial resolution of modern CT scanners and the capability for ECG gating, even the coronary arteries can be evaluated along with other structures, eliminating the need for invasive tests such as coronary angiography. MRA also provides a high level of accuracy in the diagnosis of acute aortic syndrome.
Clinical features The cardinal symptom of acute aortic syndrome is acute chest pain, which is usually projected posteriorly between the scapulae. It is typically perceived as a severe tearing or shearing pain that is maximal within seconds or minutes. Two or more peaks are known to occur, especially in patients with an aortic dissection. In this case the location of the pain will shift with the advancement of the dissection flap in the aorta and its side branches. A pulsating pain has also been described in patients with a penetrating aortic ulcer, for example. Atypical pain or a complete absence of pain have also been reported in acute aortic syndrome. Physical examination may reveal pulse and blood pressure discrepancies between the upper and lower limbs, depending on the cause. Acute aortic syndrome may also be manifested by its complications. Diagnosis may be delayed in cases that present as an acute myocardial infarction due to dissection-related displacement of the coronary arteries, for example; or as acute left heart failure due to aortic insufficiency; or as syncope due to carotid involvement, dissection, or pericardial tamponade; or even as acute abdomen due to mesenteric ischemia.
Differential diagnosis In the differential diagnosis of acute chest pain, acute aortic syndrome is the most common acutely life-threatening condition after acute coronary syndrome. Differentiation is particularly required from myocardial infarction and pulmonary embolism. The complete differential diagnosis of acute aortic syndrome should include all conditions that present with acute chest pain (or occasionally with lumbar pain). Spontaneous aortic rupture is also an important differential diagnosis.
Key points In the differential diagnosis of acute chest pain, acute aortic syndrome is the most common acutely life-threatening condition after acute coronary syndrome, ranking ahead of pulmonary embolism. Because the plain chest radiograph is normal in more than 20% of cases, swift and definitive imaging, preferentially by ECG-gated CT, provides the basis for effective patient management.
Aortic Dissection
Brief definition Aortic dissection occurs when a tear in the intima allows blood between the wall layers, causing a longitudinal separation of the intima from the media. This creates a new, false vascular lumen. The separation of the wall layers may spread in an antegrade or retrograde direction. This may lead to involvement of the aortic branch vessels, although vessels that are perfused entirely from the true lumen are rarely affected. The pressure in the false lumen may equal or exceed the pressure in the true lumen. In principle, the false lumen may remain patent, it may thrombose, it may recommunicate with the true lumen, or it may rupture into adjacent spaces such as the pericardial, pleural, or peritoneal cavity. Classification is based on the location of the reentry point or the primary intimal tear. The most widely used system is the Stanford classification, which distinguishes between type A and type B dissections (▶ Fig. 3.21). In a Stanford type A dissection, the primary intimal lesion is located in the ascending aorta (60–70% of cases); in a type B dissection, it is located distal to the origin of the left subclavian artery (30–40% of cases). In type B dissections the aortic arch and ascending aorta are not involved in the aneurysm. The reported incidence is approximately 3 per 100,000 per year in Germany and the Western World. Type A dissections have a peak age incidence in the fifth and sixth decades, while type B dissections are most common in the seventh decade. Risk factors for the development of an aortic dissection are arterial hypertension, cystic medial necrosis, vessel wall diseases such as Marfan’s syndrome, and preexisting aortic aneurysms. Iatrogenic dissections after catheter-based procedures or aortic surgery have also been described. An acute, usually symptomatic dissection is distinguished from a chronic dissection (lasting longer than 8 weeks), which is usually asymptomatic.

Fig. 3.21 Stanford classification of aortic dissection. This classification is based on the location of the primary intimal lesion. With a type A dissection it is located in the ascending aorta; with a type B dissection it is distal to the left subclavian artery. (Reproduced from Schünke M et al. Prometheus. Teaching Atlas of Anatomy: Internal Organs. Illustrated by M. Voll and K. Wesker. 2nd ed. Stuttgart: Thieme Medical Publishers; 2009.)
Imaging signs The chest radiograph often shows mediastinal widening with a widened aortic silhouette. The PA radiograph in aortic dissection may occasionally show a separation of aortic-wall calcifications from the outer aortic silhouette by more than 1 cm in the aortic arch. Pleural effusion is a consistent radiographic finding. The chest film may also show no abnormalities at all. Transthoracic and transesophageal echocardiography can visualize the dissection, although the sensitivity of transthoracic echocardiography is no better than 83% in various studies. Moreover, neither of these echocardiographic techniques can define total extent or detect involvement of the major branch vessels. Thus, CTA and MRA are the modalities of choice for a presumed aortic dissection and have replaced invasive angiography as the gold standard.
Caution
When using CTA or MRA, especially with a type A dissection, note that motion artifacts at the aortic root and in the proximal ascending aorta can mimic a dissection. It is therefore best to use ECG gating. This technique is also very helpful for detecting possible involvement of the coronary arteries.
Sectional imaging typically shows a membranelike flap separating the true lumen from the false lumen (▶ Fig. 3.22). The thickness of the intimal flap increases over time, but the flap in an acute dissection may be relatively thin. The true lumen can usually be traced owing to its continuity with the undissected portion of the aorta. An intraluminal thrombus may identify the false lumen. Because of pressure differences, the true lumen is smaller than the false lumen in approximately 90% of cases. Delayed imaging at 90 seconds may show delayed enhancement of the false lumen. Additionally, even unenhanced CT will consistently show calcifications that are displaced inward from the vessel wall. Unlike intramural hematoma, these calcifications may be arranged in a straight line if they lie on the intimal axis. Sectional imaging may also identify an entry point into the false lumen and perhaps a reentry point into the true lumen, as well as the involvement of aortic branch vessels. The sensitivity and specificity of CT and MRI in the diagnosis of an aortic dissection are reported as approximately 95% and 100%, respectively.

Fig. 3.22 Stanford type A aortic dissection. CTA can diagnose an aortic dissection and also define its extent. (a) The intimal flap starts in the ascending aorta and separates the true lumen from the false lumen. (b) The false lumen is somewhat less opacified than the true lumen, particularly in the descending thoracic aorta. (c) The dissection extends into the right common carotid artery. (d) Involvement of the visceral arteries is also clearly displayed.
Note
Besides confirming the diagnosis and providing a classification, imaging should also document the precise extent of the aortic dissection. It is also important to document the involvement and perfusion of the side branches (see ▶ Fig. 3.22), as this will have therapeutic implications.
In treatment planning, it is important to distinguish a dynamic obstruction due to collapse of the true aortic lumen or obstruction by an intimal flap from a static obstruction in which the intimal flap enters a branch-vessel origin without a reentry point. Dynamic obstruction is treated by interventional aortic fenestration, while static obstruction is treated by placement of an intravascular stent.
Clinical features The clinical presentation of an acute aortic dissection is the same as in acute aortic syndrome. Syncope occurs in 5 to 10% of patients with an aortic dissection. This is more common in patients with a type A dissection than patients with a type B. Syncope points to complications of the dissection such as pericardial tamponade or the involvement of supra-aortic arteries. Spinal cord ischemia may also occur (2–10% of cases), especially in type B dissections, due to the interruption of essential intercostal arteries. Other possible manifestations are pulse deficits and nerve lesions. The spontaneous course of a symptomatic type A dissection is associated with a mortality rate of 1 to 2%, which is increased by complications such as pericardial tamponade or involvement of supra-aortic branch vessels. An acute, symptomatic type B dissection has a 2-week mortality rate of approximately 10%. Advanced age, aortic rupture, shock, and decreased blood flow to dependent areas are independent predictors of increased early mortality. If the patient survives the acute phase, the dissection may resolve spontaneously with some residual wall thickening, or the false lumen may undergo complete thrombosis. However, the majority of dissections show persistent (partial) perfusion of the false lumen with or without aneurysmal dilatation of the false lumen. Over time, up to 20% of dissections may undergo progressive dilation and rupture even in the chronic stage. Type A dissections are treated by open surgery, while acute type B dissections can be treated interventionally. A chronic dissection is generally treated surgically or with a stent graft if it shows aneurysmal progression (1 cm growth/year) or if the aneurysm diameter is greater than 5.5 cm.
Differential diagnosis Differentiation is mainly required from intramural hematoma and penetrating aortic ulcer. Additionally, motion artifacts in the aortic root and ascending aorta or a high pericardial reflection site can mimic a type A dissection (pseudodissection). A mural thrombus in a fusiform aortic aneurysm may also be misinterpreted as an aortic dissection with a thrombosed lumen.
Key points Symptomatic aortic dissection is a condition with a high mortality. According to the entry point of blood into the false lumen, aortic dissections are classified as type A (involvement of the ascending aorta or aortic arch) or type B (distal to the left subclavian artery). Imaging tasks are confirmation of diagnosis, classification with identification of the entry point and possible reentry point, determination of extent, and detection of complications.
Intramural Hematoma
Brief definition Aortic intramural hematoma, also known by the erroneous term “atypical dissection,” results from a spontaneous rupture of the vasa vasorum of the aortic media with subsequent bleeding into the vessel wall (▶ Fig. 3.23). Unlike an aortic dissection, which is considered a precursor of this condition, an intramural hematoma forms without disrupting the intima. An intramural hematoma may extend over the complete length of the aorta. The Stanford classification distinguishes type A, which involves the ascending aorta, from type B, which does not; 60% of intramural hematomas are assigned to type A. 15 Starting from an intramural vascular rupture, the process may spread and progress to an aneurysm by weakening the aortic wall or to a dissection by tearing the intima. The thickness of the intramural hematoma appears to be a predictor of the likelihood of progression to a dissection: hematomas thicker than 11 mm are more likely to form a dissection. The hematoma may also resolve spontaneously over a period of months, especially if it involves only the descending aorta.

Fig. 3.23 Intramural hematoma. Schematic representation. An intramural hematoma results from a spontaneous rupture of the vasa vasorum. This leads to intramural bleeding that weakens the aortic wall and increases the vascular diameter. In contrast to a dissection, the intima remains largely intact.
Imaging signs The chest film findings of intramural hematoma, like those of aortic dissection, are nonspecific and unrewarding. Unenhanced CT in the acute phase shows eccentric thickening of the aortic wall with a crescent-shaped, acutely hyperdense area corresponding to the intramural hematoma (▶ Fig. 3.24). The hematoma shows fading density over time, and by one week it may already be isodense or even slightly hypodense to the aortic lumen. Wall calcifications may be displaced inward but retain their circumferential structure. On CTA, an intramural hematoma is distinguished from a dissection or penetrating aortic ulcer by the absence of a visible intimal tear, dissection flap, or outpouching of the aortic wall. Intramural hematoma is also clearly visualized by MRI, which displays the typical morphological changes described above. The age of an intramural hematoma can estimated from the signal intensity of the intramural hemorrhage:
The blood in a fresh intramural hematoma appears hyperintense in T2W GRE sequences and isointense in T1W sequences.
The signal intensity changes if the hematoma is more than 7 days old: T2W images show intermediate signal intensity while the blood appears hyperintense in T1W images due to the presence of methemoglobin.

Fig. 3.24 Acute intramural hematoma. Typical appearance of eccentric thickening of the aortic wall with separation of the calcified plaques from the outer aortic contour. A hyperdense crescent-shaped component ([a], arrows) is a characteristic finding on unenhanced scans in the acute phase. It represents the fresh intramural hematoma. (a) Unenhanced CTA. (b) Contrast-enhanced CTA. (Reproduced with kind permission of R. Fischbach, Hamburg, Germany.)
Absence of change in signal characteristics may indicate active bleeding.
Note
Besides a change in density or signal intensity, intramural hematoma may exhibit a number of other changes over time: the hematoma may become thinner, and it may dissolve completely over a period of several months. In complicated cases, the hematoma may progress to a frank dissection or an aortic aneurysm, each of which is associated with typical complications. Regular follow-up imaging is essential, therefore.
Clinical features The clinical presentation of an intramural hematoma resembles that of an acute aortic dissection. Chest pain is more common with an intramural hematoma of the ascending aorta, whereas back pain is more suggestive of an intramural hematoma of the descending aorta. An important clinical distinction is that malperfusion syndromes are less common with aortic intramural hematoma than with aortic dissection. Syncope, anterior spinal artery syndrome, hoarseness, and diminished pulses are also uncommon. Pericardial or pleural effusion may occur but is not typical. The diagnosis of intramural hematoma cannot be excluded from clinical findings alone, however, and 5 to 20% of all patients with a clinically suspected dissection are found to have an intramural hematoma. Consequently, sectional imaging of the entire aorta should be performed in all suspected cases. The clinical course is highly variable and difficult to predict. Treatment is controversial; type A intramural hematomas are usually treated surgically, while type B hematomas are managed conservatively whenever possible.
Differential diagnosis The first differential diagnosis to be considered is aortic dissection. Also, the layering phenomenon in an intramural thrombus in an aortic aneurysm is easily mistaken for chronic intramural hematoma, which may also be associated with aortic dilatation. In most cases, however, chronic hematoma can be diagnosed from the history and clinical presentation. Aortitis is also associated with wall thickening but, unlike an intramural hematoma, does not enhance after IV contrast medium administration.
Key points Intramural hematoma results from a spontaneous rupture of the vasa vasorum in the aortic wall with subsequent intramural hemorrhage. In contrast to a classic dissection, the intima remains intact. The two entities cannot be distinguished from each other clinically and require sectional imaging, usually by CT imaging of the entire aorta. CT typically shows crescent-shaped wall thickening, which is hyperdense to the aortic lumen in the acute phase and becomes isodense over time. Given the potential for complications in the form of dissection or aneurysm formation, regular imaging follow-up is essential.
Penetrating Aortic Ulcer
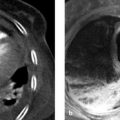
Brief definition A penetrating aortic ulcer is defined as an ulceration that has penetrated through the internal elastic lamina into the media of the aorta. It is associated with a variable amount of hematoma in the aortic wall. 15 A penetrating aortic ulcer typically results from the ulceration of atherosclerotic plaque in patients with severe aortic sclerosis. In the early phase the lesion is confined to the intima and is usually painless. A penetrating aortic ulcer may lead to erosion of the vasa vasorum by the ulcer, forming a painful intramural hematoma. This condition has a poorer prognosis than a simple penetrating aortic ulcer. The descending thoracic aorta and abdominal aorta are predominantly affected. Patients with a penetrating aortic ulcer are typically over 70 years of age.
Imaging signs Penetrating aortic ulcers appear on sectional imaging and invasive angiography as a circumscribed, craterlike outpouching in the aortic wall. They may be accompanied by local intramural hematoma but, unlike a typical intramural hematoma, a penetrating aortic ulcer appears as a localized lesion that is generally confined to the descending or abdominal aorta. Variable thrombus deposition may be found on the wall of a penetrating aortic ulcer. As a general rule, penetrating aortic ulcers are associated with severe atherosclerosis (▶ Fig. 3.25).

Fig. 3.25 Penetrating aortic ulcer in a 75-year-old man presenting clinically with back pain. (a) CTA. A large ulceration, disrupted posteriorly, with a small amount of mural thrombus is demonstrated in the visceral segment of the abdominal aorta. (b) Sagittal reformatted image shows the mushroomlike appearance of the penetrating aortic ulcer and the underlying atherosclerosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree