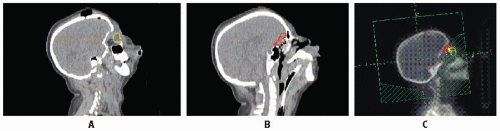
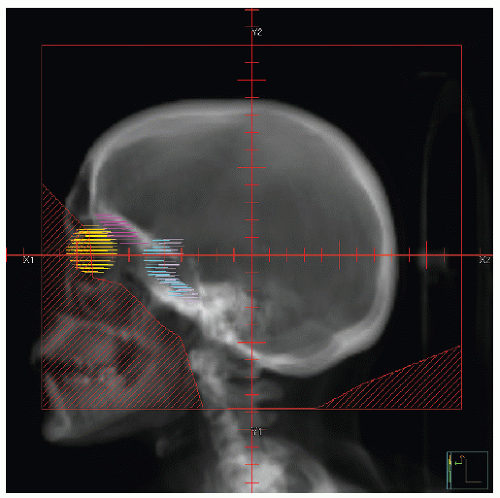
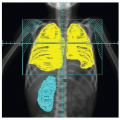
Early studies incorporated 5-12 Gy craniospinal irradiation (CSI) following the observation that CNS relapse was the dominant event once chemotherapy prolonged “hematologic remission” beyond 6-12 months (
32). CNS preventive therapy evolved through serial studies at SJCRH and in Cancer and Leukemia Group B. CSI or cranial irradiation (CrI) to 24 Gy (the latter combined with intrathecal methotrexate [IT-MTX]) reduced the incidence of CNS
relapse from >60% to <5-10% (
32,
35). Children in hematologic remission randomized to preventive CSI (24 Gy) or equivalent therapeutic CSI at the time of overt CNS relapse showed strikingly higher event-free and overall survival with preventive therapy (
36). In addition, children cured following a CNS relapse had significantly greater functional deficits (e.g., seizure disorders and cognitive deficits) (
37). Subsequent trials proved the relative equivalence of 18 Gy (at 1.5-1.8 Gy per fraction) and 24 Gy (similarly fractionated) for preventive CrI (
38,
39,
40,
41).
Other approaches to preventive CNS therapy were developed in the 1970s, with higher-dose systemic MTX and increased IT chemotherapy (
39,
40,
42,
43). Trials over the past 10-15 years have limited use of preventive CrI by progressively defining cohorts at higher risk for CNS relapse most likely to benefit from CrI—balancing the proven efficacy of CrI against radiation-related toxicities (in particular neurocognitive deficits and secondary neoplasms) and toxicities associated with the added CNS exposure to IT and systemic MTX (
2,
15,
22,
44,
45,
46,
47,
48). The Dana-Farber Cancer Institute (DFCI) Childhood ALL Consortium Protocol 95-01 included preventive CrI for 60% of children: all high risk (defined by WBC > 50,000/mL, T-cell ALL, Ph+ or t(9;22)) and, by randomization, half the standard risk (randomized to CrI vs. more intensive IT chemotherapy); girls with WBC < 20,000/mL were
assigned to the IT arm. The high-risk cohort was randomized between 18 Gy in 10 fractions (180 cGy once daily) or in 20 fractions (90 cGy twice daily). The EFS and OS at 5 years were 82% and 90%, respectively; the rate of CNS relapse was 3% overall and 0.6% as an isolated event. CrI was associated with zero isolated or combined CNS relapses in the standard risk group; the IT group showed only <2% combined and 1% isolated CNS failures (
2). There were no differences in neurocognitive function when intensive IT was compared to CrI
18 Gy (
49). The German-Austrian-Swiss ALL-BFM-95 study limited preventive CrI (dose = 12 Gy) to those with T-cell ALL and high-risk disease above 1 year old (high early MRD, t(9;22) or
BRC-ABL, or t(14;11) or
MLL-AF4). Compared to the earlier ALL-BFM-90 trial, CrI was used in 10% instead of more than 50% of cases; even at higher thresholds of CrI, the BFM-95 therapy resulted in EFS of 80% and OS of 87% at 6 years. The rate of CNS relapse was 1.8% isolated and 4.1% overall (
50). Comparing the earlier BFM-90 cohort (medium risk, age >1 year with pre-B-cell disease) to equivalent cases that did not receive CrI on the BFM-95 trial, EFS was the same, but the overall (4.4% vs. 1.9%) and isolated CNS failure rates (2.2% vs. 0.5%) were statistically superior, if perhaps not clinically meaningful, following CrI
12 Gy. Isolated CNS failures occurred early and were associated with poorer outcome after therapy for CNS relapse, but represented a low percentage of events (
50). The investigators noted not only the superiority of CrI, but also the higher rates of secondary carcinogenesis. Comparative outcome has also been addressed following CNS relapse in the BFM-95 trial; 58% secondary survival was observed 6 years after relapse and secondary brain tumors are yet at zero (3-4 years following CSI) (
15). St. Jude Total XV trial was designed specifically to test eliminating preventive CrI and “therapeutic” CrI (i.e., for those with CNS 3 involvement at diagnosis), the first major US prospective trial assessing intensive IT and systemic chemotherapy to totally replace CrI (
14). The impetus was based largely on the high rate of secondary neoplasms identified in the preceding St. Jude Total XII trial (12-20%) (
28,
51,
52,
53) (
Fig. 2.4). Trials XIIIA and XIIIB had shown CNS relapse rates of 1.2% and 1.7%, respectively, with thresholds for preventive CrI resulting in 22% and 12% of participants receiving CrI, respectively (
24). Total XV trial was powered to document a low rate of CNS relapse even in the highest-risk category, defined in the key Italian BFM report as T-cell ALL with WBC > 100,000/mL (
14,
54); only children with persistent CNS disease at conclusion of induction therapy were scheduled for CrI. On comparing the highest-risk cohort regarding CNS disease in Total XV trial with the 12% of similarly presenting patients who had received CrI on the earlier trial, EFS was statistically equal or better in the Total XV group without CrI (
14). Despite the high overall EFS and low rate of CNS relapse, one must note the low rate of overall disease control (43%) in the CNS 3 small cohort with initial disease in a without use of CrI in this presentation (
14,
22,
55).
Table 2.1 summarizes some of the major series reporting therapeutic approaches over the past 10-15 years. In sum, it is apparent that standard risk ALL can be treated without CrI (
15,
38,
39,
41,
43). The group for which CrI may be beneficial is the cohort with T-cell ALL and presenting with WBC greater than 100,000/µL. Approximately 20% of children with T-cell ALL (or 2% of all children with ALL) present with high WBC and seem to benefit from preventive CrI (
15,
44). Because HD-MTX and IT-MTX have been associated with neurologic sequelae, there is also interest in considering
further CrI dosage reduction (12 Gy/8 fractions in the more recent BFM studies) (
39).