Fig. 7.1
Standard liver measurements and liver segments: (a) Liver anatomy with liver segments (I–VIII), relevant liver vessels for anatomical classification (portal vein, hepatic vein, inferior vena cava) and standard planes for US measurements, particularly right anterior axillar line (AL), right and left middle clavicular line (r/lMCL) and medially positioned sterna line (STL). Two respective normal sagittal views with important reference structure (upper pole of right kidney for AL, abdominal aorta for STL) given. (b) Standard image appearance of typical liver sections: the transducer position and orientation given in the schematic drawing, with respective US images
NOTE: Due to complexity of the liver, documentation and measurements in standardised sections are essential. For identification of section – include other key structures on image, particularly important for comparison during follow-up.
7.2 Normal Findings
7.2.1 Structure
Liver has larger right and smaller left lobe, internal architecture classified by liver segments (Fig. 7.1).
Parenchyma – homogenous echo of medium echogenicity, contour and borders as well as sharp and smooth margins. Size varies with age, particularly height – measurements correlated with normograms (Table 7.1).
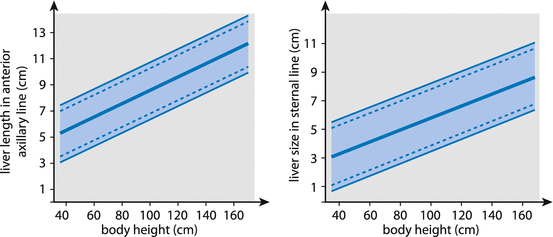
Table 7.1
Normal liver size in relation to age/height

NOTE: Physiologically left liver lobe is much larger in neonates than in older ages; it gradually shows relative decrease in size after closure of ductus venosus.
7.2.2 Ligaments
Ligamentum falciforme hepatis, ligamentum teres hepatis and ligamentum triangulare hepatis fix liver within abdomen, usually only seen with ascites.
7.2.3 Hepatic Veins (HV)
Converge cranially to drain into subdiaphragmatic inferior cava vein (ICV) or into right atrium. usually three main liver veins (+ caudate vein); additional veins or varied insertion at different levels of intrahepatic ICV exist as normal variants. Size may vary with respiration and intravascular volume – usually exhibit smooth border and straight course, with relatively thin low echogenicity wall.
7.2.4 Portal Vein (PV)
Enters at hepatic hilus, should not show tapering (a sign of portal hypertension). Branches between left and right PV in liver centrally, left PV shows focal ectasia (Rex recessus, sinus venosus) at area of former insertion of umbilical vein draining via venous duct of Arantii (ductus venosus) to right atrium during fetal circulation. Wall slightly more echogenic, partially increased by periportal structures (bile ducts, accompanying arteries). Periportal region gets enlarged and more echogenic in various conditions or may appear prominent with decreased echogenicity of liver parenchyma.
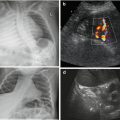
NOTE: In neonates (during first weeks of life), communication may persist between sinus venous and ICV/right atrium (“physiologically persistent ductus venous” Fig. 7.2a) – should obliterate spontaneously. Persistent ductus venosus usually indicates underlying liver disease with increased peripheral liver resistance. Umbilical vein may be visible in neonates physiologically, but shows no flow – consequently thromboses and vanishes; persisting (inverted) flow in umbilical vein – sign for portal hypertension and consequent portosystemic shunt. A nonfunctioning umbilical vein catheter may end in this vein – actively search for it (Fig. 7.2b).
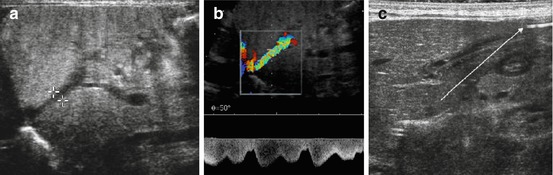

Fig. 7.2
Neonatal liver: (a) Physiologic persistent ductus venosus (+ +). (b) Flow and patency of neonatally persistent ductus venosus depicted by CDS – flow direction documented by Doppler trace. (c) Still visible umbilical vein with catheter (arrow)
7.2.5 Hepatic Artery (HA)
Can be followed from its origin at coeliac trunk parallel to PV to branching into left and right HA.
Usually CDS enables easy identification and differentiation from other tubular structures, particularly in liver periphery, where differentiation of HA from (dilated) intrahepatic bile ducts is otherwise difficult.
NOTE: Numerous normal variants in HA anatomy, e.g. accessory left HA from coeliac trunk or gastric artery, separate origin of right HA from superior mesenteric artery. CDS particularly valuable for assessing these anatomic variants.
7.2.6 Gall Bladder
Positioned on lower surface of liver, centrally close to hilus on right side. Usually shows thin wall, contents unechoic. Size can vary with time since feeding. Best seen in subcostal oblique view in MCL.
NOTE: An empty or poorly filled gall bladder, particularly if relatively large when filled, may show a pseudothickened wall (Fig. 7.3a).
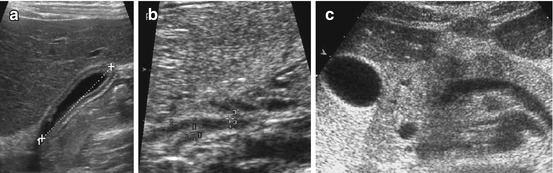

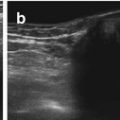
Fig. 7.3
Bile system: (a) Normal, nearly empty gallbladder (+…..+) with pseudothickening of wall. (b) Normal common hepatic duct (3, 2+ +), confluence with cystic duct (1+ +). (c) Axial section shows pancreatic portion of prominent common bile duct
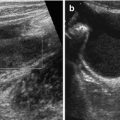
7.2.7 Common Bile Duct (Commonly Addressed as Hepato-Choledochal Duct)
Usually crosses main PV and runs through head of pancreas to papilla/ampulla of Vater.
NOTE: Duct (even ductus cysticus) often visible using high-resolution linear transducers (Fig. 7.3b, c).
7.2.8 Intrahepatic Bile Ducts
Course parallel to PVs – usually only depicted centrally or if dilated.
7.2.9 Doppler Findings
7.2.9.1 Hepatic Veins (HV)
Show bi- or triphasic undulating flow pattern, bidirectional flow direction. Undulation caused by respiration and heart cycle (Fig. 7.4a). Absence of typical pattern usually indicates either increased liver resistance or increased right atrial pressure/volume overload.
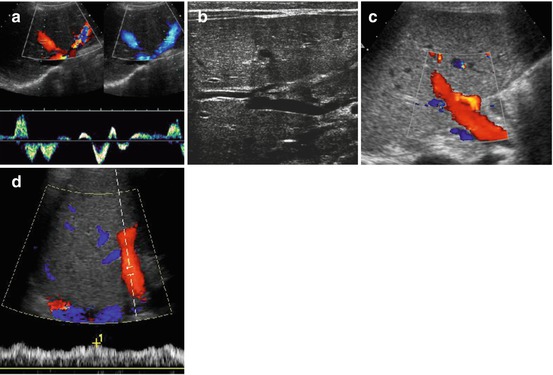

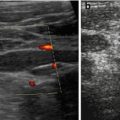
Fig. 7.4
Liver vessels: (a) Hepatic veins, bidirectional undulating flow demonstrated by CDS (either red or blue signals indicating two flow directions during respiratory cycle) with cardiac and respiratory modulation also demonstrated by spectral flow analysis. (b) Main portal vein entering liver: note no significant variation in diameter. (c) Portal vein flow on CDS (red colour signals): note adjacent hepatic artery with faster flow in same direction (coded in orange). (d) Duplex-Doppler flow profile confirms normal hepatopetal portal flow +1 indicates maximum velocity
NOTE: Flow profiles may vary within the three main veins, also depends on point of insertion and manoeuvres used for visualisation (such as breath holding). Undulation must always persist; bidirectional or triphasic pattern may physiologically be absent.
7.2.9.2 Portal Vein (PV)
Usually shows constant flow into liver with some mild respiratory modulation (Fig. 7.4b–d).
Flow velocities vary with age (Table 7.2a) and fasting status.
Table 7.2
Normal values for liver vessel flow
(a) Normal flow in portal vein | |||||
Rule of thumb: above 20 cm/s in non-fasting child (general lower cut-off value): | |||||
With some respiratory undulation | |||||
Flow pattern and – to a lesser degree – flow velocities influenced by respiration: | |||||
More undulating flow in earlier years than later in childhood. | |||||
In healthy term neonates peak portal blood flow may double 15 min after feeding: | |||||
Fasted = 24 ± 1.3 cm/s after feeding = 35.9 ± 2.4 cm/s | |||||
(b) Age-dependent flow velocities in hepatic artery | |||||
Age (years) | PV diameter (mm) | PV velocity (cm/s) | PV flow (ml/min) | HA velocity (cm/s) | HA RI (%) |
0–5 | 3.5 ± 2 | 9.1± | 70 | ?a | ? (85 ± 15)a |
6–12 | 6.3 ± 2 | 13.4± | 250 | ? (50–100)a | ? (60 ± 10)a |
>12 | 7 ± 2.6 | 14.6 ± 5 | 380 | 70–120 | 60 ± 4 |
Average values are not applicable, as velocities are very low in neonates (around 40 cm/s), even lower in preterm babies, and increase relatively quickly to values similar to older children – HA hepatic artery, PV portal vein | |||||
aNo established normal values – the numbers given for orientation are from individual reports and own experience, particularly in neonates | |||||
(c) Hepatic vein flow profile | |||||
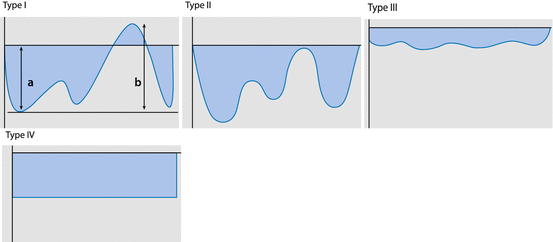 | |||||
Schematic drawing of typical flow patterns of hepatic veins (Types I–IV), and how elasticity index (EI) is calculated (EI = a/b %) (d) typical flow patterns of heaptic veins | |||||
Type I | Normal | Triphasic, bidirectional flow | EI ~ 120 % | ||
Type II | Unspecific | Triphasic, monodirectional flow | EI ~ 90 % | ||
Type III | Dampened | Tonodirectional, but still modulated flow | EI ~ 40 % | ||
Typ IV | Severely impaired | Monodirectional, uniform, unmodulated flow | EI ~ 0 % | ||
NOTE: Also intrahepatic PV branches should be assessed to show patency of at least main right and main left PV. Flow velocity measurements strongly rely on proper angle correction and good insonation angle (<60°).
7.2.9.3 Hepatic Artery (HA)
Less often important
Systolic velocity varies with age and nutritional status (Table 7.2b), as does diastolic velocity/RI
CDS essential for differentiating particularly peripheral branches from biliary structures and for proper angle correction (if flow velocity measurements taken).
NOTE: HA shows same flow direction as portal vein – only by selecting proper CDS settings (gate, filter, flow velocity) – discrimination of PV and HA feasible on CDS.
7.2.10 Special Aspects of Newborns and Infants
After closure of ductus venosus – relative increase in size of the right liver lobe – neonates have significantly larger left liver lobe than older children. Left and right liver lobes may even be similar in size
Newborns’ liver softer and more flexible – slightly rounded lower margin is physiologic
Parenchyma less differentiated and less echogenic than in older age due to reduced fibrosis and less fat – compared to renal cortex liver parenchyma echogenicity is lower
Periportal echoes less prominent
Ductus venosus can remain patent up to some weeks – seen as tubular vascular structure coursing from ectatic section of left PV to ICV/right atrium. Closes spontaneously – then seen as echogenic band (= ligamentum venosum in later age) (see Fig. 7.2a, b)
Unusual or altered flow patterns in neonates do not always indicate hepatic disease; often reflect physiologic systemic or vascular variations (e.g. patent foramen ovale, PDA, persistent ductus venosus Arantii). Interpretation of Doppler findings needs to be made with care and respect to all other clinical and laboratory data as well as imaging findings; sometimes additional assessment of abdominal aorta, renal or cerebral vessels, heart and other large vessels necessary for deciding on origin of unusual flow findings
7.3 Pathology of the Liver
7.3.1 Congenital Changes and Normal Variance
7.3.1.1 Situs Inversus (Abdominalis)
Definition
Liver on left side, no isolated liver malformation.
US Findings
Liver seen in left upper quadrant, spleen situated in right upper abdomen (potentially with multiple splenunculi – polysplenia syndrome). Liver anatomy is mirrored.
7.3.1.2 Butterfly or Midline Liver
Definition
Often associated with other (systemic) malformations and syndromes (e.g. ambiguous situs, polysplenia syndrome, asplenia, gastroschisis and omphalocele).
US Findings
Butterfly-shaped liver positioned medially, often hilus structures in unusual position and relation, more difficult to find and identify. Left and right liver lobes often of similar size. HVs drain directly into right atrium or into hemiazygos continuation of interrupted ICV.
7.3.1.3 Hypoplasia/Atrophy of Left Liver Lobe and Other Variations
Hypoplasia is usually a consequence of intrauterine events – not congenital or hereditary, only conatal malformation.
Other variants are diaphragmatic bump filled with liver, variations associated with diaphragmatic hernia and liver up-position, variations associated with gastroschisis and omphaloceles.
Many variations both of arterial and venous anatomy are most identified by proper technique using CDS. Have little other implication – unless liver surgery or transplantation planned.
7.3.2 Inflammatory Conditions
7.3.2.1 Hepatitis
Definition
Mostly viral in origin. May occur in any time of childhood, even neonatally. Shows large variance in its course and outcome.
During its course there may be fibrosis, other diffuse hepatopathies, enlargement or shrinkage, eventually cirrhosis or secondary abscess formations.
US Findings
Unspecific – liver normal or slightly enlarged. Usually surface remains smooth, lower margin slightly rounded. Echogenicity more or less decreased, with normal appearance of periportal area, unless significant decrease in parenchymal echogenicity – then periportal regions become prominent. Associated oedematous changes of gall bladder wall and bed possible (DDx: cholecystitis, congestive, vasculitis such as Kawasaki disease, during intensive care).
Associated findings: enlarged lymph nodes in hepato-duodenal ligament, splenomegaly.
CDS
Hyperaemia with low RI of HA, potentially increased PV flow; in severe enlargement and thus increase in liver resistance also reduced undulation in HV.
NOTE: US does not allow definition of aetiology or discrimination between viral, bacterial, toxic, drug-induced or other forms of hepatitis/hepatopathies. Mimics exist.
7.3.2.2 Liver Abscess
Definition
Usually haematogenous, sometimes ascending through bile ducts.
Various entities – bacterial, fungus, secondary after malignant infiltration, echinococcal or other parasites (amoebiasis, ascariasis, schistosomiasis, etc.).
US Findings
Spherical or polygonal focal alteration of liver texture. If necrotic – central hypoechoic, sometimes with (floating) echoes, in complex abscess formations increased central echogenicity, fluid levels. Usually peripheral membrane and halo-like peripheral hypoechogenicity (Fig. 7.5).
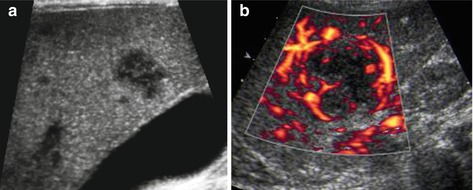

Fig. 7.5
Liver abscess. (a) Focal nodular hypoechoic disruption of liver parenchyma and several initial small lesions – in this case fungal septicaemia in oncology patient during chemotherapy (fungal abscess). (b) CDS may confirm the necrotic avascular nature and show peripheral, membrane-like hypervascularisation
Image varies with course – only subtle findings in beginning, more pronounced in later stage.
Other focal liver lesions sometimes difficult to differentiate (metastasis and infiltration in systemic haematologic disease).
CDS
Shows central avascular nature with peripheral membrane-like hypervascularisation and hyperaemia. Reactive diffuse hyperaemia of HA and PV.
If associated with ascending condition, gas or air bubbles seen in peripheral bile ducts – causing small echoes with reverberation and twinkling on CDS (aerobilia). Spectral trace shows blip-like short high-amplitude partially bidirectional signals.
Special Entities:
Echinococcal/hydatid disease: manifest as cystic liver lesions; different appearances depending on age and stage (Type I –Type IV, Gharbi classification used most commonly) (Table 7.3) (Fig. 7.6). In children Type I and III commonly seen (younger manifestation). Differentiation of Type I lesion from other hepatic cysts difficult. US used for puncture and therapeutic alcohol/drug instillation
Table 7.3
Hydatid disease – grading system (Gharbi classification)
Type I: pure simple cystic fluid collection
Type II: cystiform fluid collection with split, nodular-irregular wall; may contain echoes in central compartment
Type III: cyst with complex fluid collection and internal septae
Type IV: cyst with heterogenous echo pattern, potentially beginning calcifications
Type V: cyst with reflecting thick walls (mature/old form), often has calcified walls
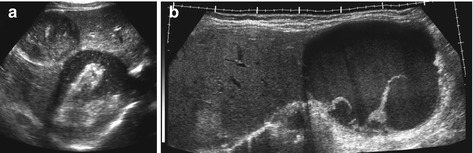
Fig. 7.6
Echinococcus: (a) Two hydatid cysts with complex inhomogenous content (stages 3–4). (b) Extended view delineates huge hydatid cyst with slightly complex fluid and septations in left liver lobe (stage 3)
Other causes of abscess: fungus, amoebiasis, ascariasis and schistosomiasis. Appearance unspecific – with more or less target appearance; sometimes linear echogenic structures demonstrated with ascariasis, or echogenic portal tracts with hepatomegaly in schistosomiasis, may aid diagnosis
7.3.2.3 Granulomatous Disease
Manifests as single or multiple hypoechoic lesion, usually representing granuloma (potentially secondary abscess formation). Similar appearance as cat scratch disease (or focal lymphoma).
US nonspecific: diagnosis made by laboratory finding, history and potentially fine needle aspiration/biopsy. Similar appearance seen in tuberculosis, sarcoidosis, metastatic disease, lymphoma or in patients with (acquired) immune deficiency syndromes (the latter often with very mixed liver manifestation).
7.3.2.4 Role of US
Nonspecific in inflammatory liver conditions
May be useful initially for narrowing down DDx or excluding other conditions that may cause similar symptoms
Used for follow-up, particularly in chronic conditions
Used for guided diagnostic punctures/therapeutic drainage/drug instillation
Additional clinical and historical data, serologic diagnoses or search for pre-existing or causative conditions always essential
7.3.3 Other Parenchymal Liver Disease
7.3.3.1 Hepatopathy
Definition
Nonspecific finding, seen in a number of conditions such as secondary to drugs and treatment (drug induced/toxic damage) or secondary to systemic or metabolic disease. May be restricted to liver (e.g., Wilson disease, Gilbert syndrome) or systemic (e.g., glycogen storage disease, tyrosinaemia).
US Findings
Liver usually slightly enlarged with slightly increased echogenicity, may have some inhomogenicity of parenchyma with a more or less nodular pattern, without interruption of normal straight and smooth vascular architecture.
Fatty Liver/Steatosis
Definition
Rare in early childhood – mostly secondary to pre-existing liver condition or liver damage.
Increasingly observed in adolescence due to obesity.
Secondary to other systemic disease/treatment or drug induced.
US Findings
Large liver, maintained smooth surface, but lower margin rounded
Echogenicity increases, structure commonly remains homogenous – focal steatotic areas usually occur along PV branches, but also elsewhere; inhomogenous steatosis possible, as well as areas of non-steatosis (Fig. 7.7a)
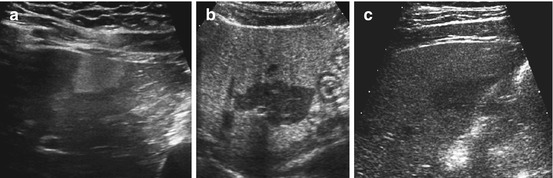
Fig. 7.7
Steatosis (focal, non-steatosis, etc.): (a) Focally increased echogenicity without disruption of contour or structure, in focal liver steatosis. (b, c) Fatty liver with increased echogenicity, focal darker non-steatosis area with consequently regional normal liver echogenicity
Due to high attenuation, there is decreased penetration of sound, increasing signal loss and noise in deeper compartments. Sound attenuation used for grading severity of steatosis
Potentially poor HV visibility
NOTE: Focal non-steatotic areas of normal tissue appear like focal liver lesions in fatty liver (Fig. 7.7b, c). Commonly of lower echogenicity, spherical or polygonal shape, often in segment IV – do not show any change of contour or alter course of vessels, which helps for DDx. Occasionally regional adenomatous hypertrophy of normal liver may occur (particularly in children after chemotherapy, who also may develop liver adenoma).
Liver Congestion
Definition
Occurs in conditions with increased right atrial pressure (secondary to congenital cardiac malformations, other cardiac disease, chronic respiratory insufficiency with increased intrapulmonary pressure such as cystic fibrosis), and secondary to intensive care with volume overload and increased right atrial pressure.
US Findings
Enlarged liver, initially reduced echogenicity
In longer duration/chronic state – increased echogenicity
Smooth surface, rounded lower margin, dilated HV that can be followed into liver periphery and showes reduced or lack of size modulation
Ascites, splenomegaly, omental thickening and oedematous thickening of congested bowel wall – secondary signs
IVC (and often LV, too) usually enlarged with reduced change in calibre during respiratory and cardiac cycle
CDS
Pronounced flow undulations triggered by atrial contractions seen into liver periphery. Particularly seen secondary to tricuspid valve insufficiency. Reduced respiratory modulation, similar phenomena seen in IVC. Depicted and documented by CDS, but lack of diameter change during respiratory and cardiac cycle nicely documented by M-Mode.
7.3.3.2 Liver Fibrosis
Definition
Either congenital genetic condition (e.g. ARPKD) or consequence of chronic conditions (cholestatic/inflammatory). Rarely isolated entity or disease.
US Findings
Initial/mild manifestation – not visible
With increasing severity and duration – increasing echogenicity of periportal areas with narrowed and potentially irregularly shaped peripheral PV branches – HV stays normal
Increased vascular resistance, increased size of HA – eventually leading to biliary cirrhosis and portal hypertension with all its findings (see below)
In underlying bile duct conditions respective changes seen (e.g. irregularity or dilatation of peripheral bile ducts). The very bright echoes of periportal fibrotic areas may produce a “starry sky” appearance of the liver (Fig. 7.8).
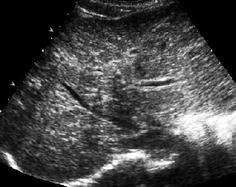
Fig. 7.8
Liver fibrosis – “starry sky” appearance. Strikingly increased periportal field echogenicity with irregular structure from biliary fibrosis
7.3.3.3 Cirrhotic Liver
Definition
Main reasons in childhood: bile duct hypo-/aplasia, extrahepatic bile duct stenosis, α1-antitrypsin deficiency, various metabolic diseases (Wilson disease, tyrosinaemia, cystic fibrosis, etc.), chronic intoxication (e.g. copper), drug induced, after hepatitis, liver fibrosis/choledochal cysts, Caroli syndrome and other ciliopathies.
US Findings
Several states during course of disease (Fig. 7.9):
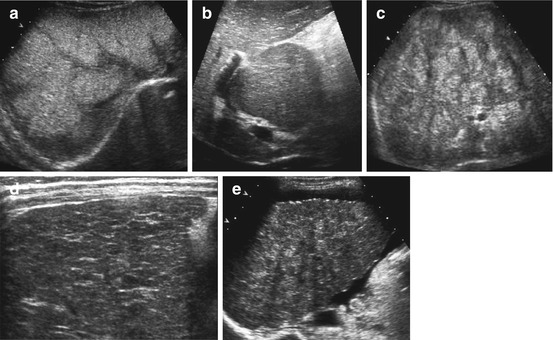

Fig. 7.9
Liver cirrhosis: (a) Diffusely altered echogenicity in a patient with alpha-1 antitrypsin deficiency. (b) Hypertrophy of caudate lobe in cirrhosis. (c) Severe macronodular cirrhosis in an adolescent with cystic fibrosis and biliary cirrhosis. (d) Micronodular alteration of liver parenchyma in a neonate with thyrosinaemia – indicating early liver cirrhosis. (e) Irregularly shaped liver surface and ascites in severe liver cirrhosis in an adolescent awaiting liver transplantation
Initially increasing size, no other specific findings
Then decrease of liver size. Irregular surface (varies with macro- and micronodular forms), convex shape of very sharp lower margin, increased inhomogenous echogenicity with reduced sound penetration, patchy parenchymal structure with focal nodular regeneration
Caudate lobe commonly spared – develops compensatory hypertrophy, becoming spherical and of lower echogenicity (See Fig. 7.9b)
HV and eventually PV become tapered, narrowed and intrahepatically diminished – with irregular course and margins
Secondary chronic changes: decreased size of gall bladder with thickened wall, splenomegaly, ascites and other findings associated with portal hypertension
CDS
Essential for showing portal hypertension (see below) – usually intrahepatic form in cirrhosis. Extrahepatic dilatation of PV with tapering at hilus as well as rarefied intrahepatic PV branches. Secondary compensatory hypertrophy of HA which becomes large and very pulsatile. Increased arterial perfusion secondary to reduced portal venous flow.
7.3.3.4 Liver Involvement in Systemic Disease
Many systemic diseases may affect liver, causing hepatomegaly and eventually cirrhosis (e.g. Niemann-Pick disease). All conditions with severe parenchymal damage develop high-arterial RI secondary to increased peripheral liver resistance associated with reduced PV flow and reduced undulations of HV flow profile.
NOTE: In most of these conditions, secondary malignant transformation and manifestation of liver carcinoma quite common – regular monitoring advisable. Nodular regeneration occurs in most of these conditions (cannot be clearly differentiated from other focal lesions such as adenoma or liver carcinoma by conventional US). Additional serologic parameters (e.g. α1-fetoprotein), IV-ce-US, dynamic CT/MR imaging or US-guided biopsy often necessary:
Cystic fibrosis
Causes cholestasis, both intra- and extrahepatically, with secondary fibrosis and biliary cirrhosis, multiple intrahepatic + common bile duct + gall bladder bile stones/calcifications, ascending cholangitis (see Fig. 7.9c).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree