Viral hepatitis
Hemochromatosis
Wilson’s disease
Glycogen storage disease
Autoimmune hepatitis
Primary biliary cirrhosis
Nonalcoholic steatohepatitis
Amyloidosis
Sarcoidosis
Diffuse metastatic disease
Focal hepatic lesions without definite benign features on imaging may prompt a focal image-guided biopsy. History of malignancy may raise the suspicion for metastatic disease; alternatively, factors predisposing to malignancy such as cirrhosis may raise suspicion for hepatocellular carcinoma. Image-guided biopsy of portal vein thrombus may be performed to evaluate tumor thrombus [10, 11]. In patients with history of lymphoma, specimens are typically submitted for flow cytometry. Immunosuppression (e.g., chemotherapy) may be an indication to submit specimens for microbiologic analysis.
Factors in the patient’s clinical history that may increase the risk of the procedure are also assessed (Table 13.2). For example, history of bleeding diathesis or easy bruising should prompt laboratory evaluation of coagulation and platelet function. Patients with severe cardiac or pulmonary disease may not be candidates for procedural sedation; the assistance of an anesthesiologist may be indicated. Pre-procedure antibiotics are typically given in liver transplant patients and prior to biliary procedures. A determination may also be made from the patient’s clinical history as to whether the patient is able to give informed consent for the procedure.
Table 13.2
Factors which may increase the risk of complication
Bleeding diathesis |
Anticoagulation or antiplatelet therapy |
Ascites |
Congestive heart failure |
Oxygen-dependent chronic obstructive pulmonary disease |
Allergy to latex, medications used in sedation, intravenous iodinated contrast |
The liver is a vascular organ, and hence, patients need to be optimized to reduce the risk of post-procedure bleeding. Along with review of the clinical history, laboratory work-up is important. A coagulation profile is often necessary, particularly in patients with history of liver dysfunction, in whom synthesis of coagulation factors may be compromised. Patients may also need to be screened for thrombocytopenia. Practices vary in the threshold levels that prompt action such as blood product transfusion. Given the risk of post-procedure bleeding, some physicians will require a pre-procedure hematocrit level to be checked and blood bank sample to be submitted.
The importance of pre-procedure imaging will depend on whether a focal or non-focal biopsy is to be performed. For non-focal liver biopsy, pre-procedure imaging is not routinely requested, although, if available, may be reviewed to assess for the presence of ascites as well as anatomical variants (e.g., course of colon relative to liver).
Pre-procedure imaging is mandatory, however, for focal hepatic biopsies for at least four reasons. First, cross-sectional imaging may be useful to exclude patients with lesions that demonstrate definite benign imaging features (e.g., hemangioma). Second, imaging will demonstrate location of the mass and help assess for distant disease. Third, pre-procedure imaging is crucial to planning a safe access route for the biopsy. Fourth, pre-procedure imaging may help the interventionalist select the best imaging modality to guide the biopsy, i.e., ultrasound, CT, or MRI.
As with other interventional radiologic procedures, pre-procedure preparation also includes withholding anticoagulants and antiplatelet agents. If sedation is to be administered, patients are generally made NPO for solid foods 8 h and for clear liquids 2 h prior to the procedure, although practices vary slightly in this regard.
Some practices routinely perform non-focal liver biopsies without procedural sedation. In such circumstances, indications for procedural sedation may include patient anxiety and pediatric age. The assistance of an anesthesiologist is rarely indicated but may be required in pediatric patients or in patients with hemodynamic or respiratory instability.
When a non-focal liver biopsy is desired, an alternative to the direct percutaneous approach is the transjugular liver biopsy. Specimens obtained by way of transjugular approach may be inferior compared to those obtained by direct percutaneous biopsy [8, 12–15]; hence, transjugular liver biopsy is generally performed in specific circumstances. Transjugular biopsy is appropriate if hepatic vein pressure gradient measurements are desired for the assessment of portal hypertension [7, 8]. It may be preferred in patients with severe coagulopathy or congenital clotting disorder, in which the risk of intraperitoneal hemorrhage is reduced by using the transjugular route [7]. Massive ascites has also been described as an indication [6, 8, 9]; however, whether ascites increases the risk of bleeding after direct percutaneous approach is controversial [16, 17]. Transjugular liver biopsy may also be used in children [6].
Contraindications
In most instances, when an image-guided liver biopsy is indicated, factors that may increase the risk of the procedure may be modified to an acceptable level. Rarely do circumstances arise in which a biopsy cannot be performed. These include uncorrectable coagulopathy, hemodynamic or respiratory instability, and lack of safe access to a hepatic lesion. In patients in need of a non-focal biopsy and who present with coagulopathy or massive ascites, a transjugular liver biopsy may be preferred [8]. Massive ascites may restrict the ability to secure safe access to a lesion or liver; pre-procedure paracentesis may be helpful.
Percutaneous Liver Biopsy
Technique (Table 13.3)
Table 13.3
General technique for percutaneous liver biopsy
1. | Clinical history is reviewed for indications, contraindications, and risk factors that may need to be modified prior to biopsy |
2. | Laboratory values are reviewed, particularly coagulation profile and platelet count; estimated glomerular filtration rate is calculated if intravenous contrast is to be administered |
3. | Prior imaging is reviewed, which is mandatory for focal liver biopsy. Putative needle entry site and trajectory are planned |
4. | Informed consent is obtained. If a hepatic dome lesion is being biopsied, discussion of pneumothorax and chest tube placement may be important |
5. | Based on review of prior imaging, patient is positioned on the table or stretcher to facilitate the biopsy, e.g., supine, prone, or left lateral oblique or decubitus position |
6. | Procedural sedation or anesthesia is administered if applicable. Patient is generally made NPO as per institutional guidelines |
7. | Preliminary images are obtained. The application of a skin grid may be helpful for CT- or MRI-guided focal liver biopsy (see text) |
8. | Needle entry site, angle of entry, and distance to lesion, if applicable, are assessed |
9. | Clean and sterilize skin using standard technique |
10. | Apply local anesthetic, such as 1 % lidocaine. Local anesthesia is optimally administered if given along the planned needle trajectory from the skin to the liver capsule. Allow one to two minutes for anesthetic to take effect |
11. | A 5-mm skin incision is made with a scalpel blade to facilitate introduction of the introducer (coaxial technique) or biopsy needle |
12. | With US, needle is advanced into the liver under real-time imaging guidance. Using CT or MRI, a stepwise approach may be used (see text). CT or MRI fluoroscopy may enable real-time visualization |
13. | Coaxial-, tandem-, or single-needle technique is performed as described in the text |
Imaging
Non-focal liver biopsies are generally performed using sonography. Focal liver biopsies may be guided using ultrasound, CT, or rarely, MRI.
Ultrasound
Ultrasound is the preferred modality for non-focal (Fig. 13.1) and focal (Fig. 13.2) percutaneous liver biopsy for several reasons. US offers real-time visualization and multiplanar capability, is relatively inexpensive, and is readily accessible. The lack of radiation is another advantage, particularly in younger patients. However, sonography is limited in its ability to identify the pleural space, which may increase the risk of post-procedure pneumothorax. Additionally, collapsed bowel may be difficult to discern.
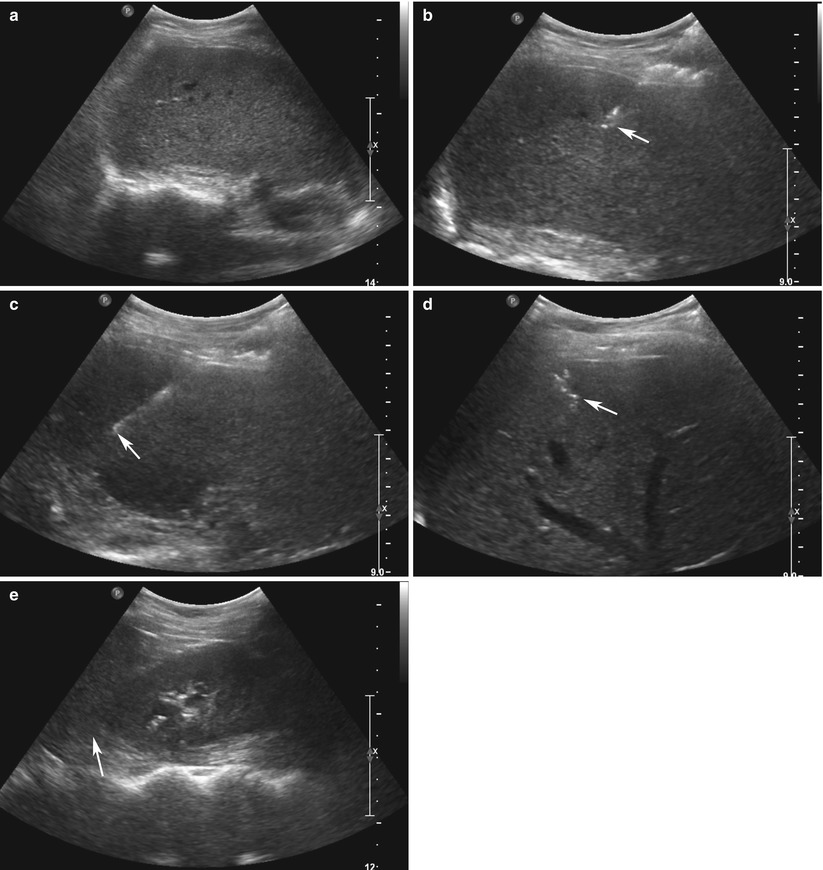
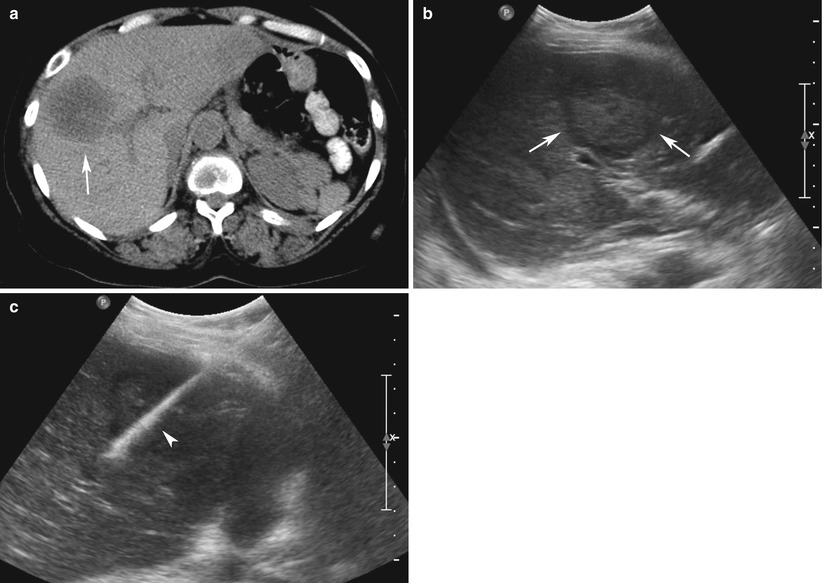

Fig. 13.1
Ultrasound-guided non-focal liver biopsy in a 51-year-old man with hepatitis C. Preliminary sonogram of the liver (a) is used to determine a clear needle trajectory to the liver. (b) A 17-gauge introducer needle (arrow), part of a coaxial needle kit, is advanced into the liver under real-time US guidance. (c) An 18-gauge core biopsy gun (arrow) is advanced into the liver (arrow) through the introducer needle. Post-biopsy sonogram of the liver at the level of the biopsy (d, arrow) and Morrison’s pouch (e, arrow) demonstrate no hemorrhage

Fig. 13.2
Ultrasound-guided focal liver biopsy in a 62-year-old man with metastases of unknown primary. (a) CT scan of the abdomen and pelvis performed with intravenous contrast demonstrates a 5-cm hypodense mass (arrow) within the right lobe of the liver. (b) Preliminary sonogram demonstrates an echogenic mass with hypoechoic rim (arrows). (c) Core biopsy needle (arrowhead) is advanced into the mass through an introducer needle. Pathology demonstrated metastatic non-small cell lung adenocarcinoma
Preliminary sonography is performed to evaluate the liver as well as the position of the gallbladder, if present. Doppler sonography is useful for the identification of intervening vessels [10, 18]. The most common practice is use of freehand technique. In general, one hand controls the transducer, whereas the other controls the biopsy needle. Some physicians prefer to use a biopsy needle guide, which attaches to the transducer. In either case, the needle can be visualized as an echogenic complex; visualization of the needle may be improved with a jiggling “in-and-out” motion [18]. Echogenic needle-tip enhancers are also commercially available.
CT
CT guidance is associated with longer procedure times, higher cost, and radiation compared to US. In addition, the needle trajectory is generally restricted to the axial plane. CT guidance is indicated in cases where the target lesion is not well demonstrated on sonography (e.g., cirrhosis) or if there is a concern for intervening structures such as bowel based on pre-procedure imaging. CT guidance may also be helpful in obese patients or patients who had a nondiagnostic result from a prior US-guided biopsy. New practitioners may also find that CT-guided biopsy is generally easier to perform than US guidance, with a shorter learning curve.
Pre-procedure images are reviewed, and a grid with radiopaque lines is placed on the patient’s skin in the area of the expected needle entry site. A preliminary CT is performed and the biopsy trajectory is confirmed (Fig. 13.3). The needle entry position on the grid is selected; the angle of entry and distance to the lesion are also assessed on the images. The skin is then marked. If an entry site is chosen that is outside the field demarcated by the grid, the skin entry site may be marked by measuring the transverse and/or craniocaudal distances of the entry site from a selected point on the grid. For example, in an anterior approach, if the planned entry site is located medial to the most medial radiopaque grid line, the distance between that line and the entry site can be measured on the images and translated on the skin. A craniocaudal component can also be assessed by counting the number of axial images above or below the CT image in which the grid is last seen and multiplying that number by the slice thickness. Alternatively, the grid position can be modified and repeat CT images obtained. After a sterile preparation and the application of local anesthetic, a needle is inserted at the marked entry site and advanced in step-by-step fashion, with intermittent CT imaging to confirm needle position. If a coaxial needle is used, the stylet may be removed prior to each CT scan to reduce needle artifact that may obscure the lesion (Fig. 13.4).
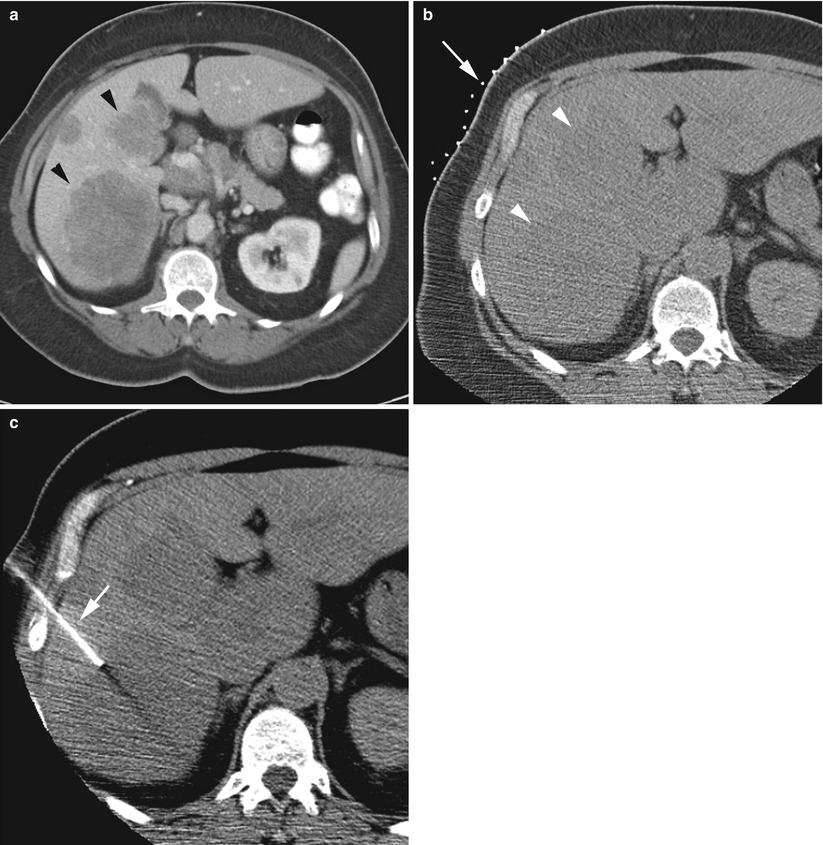
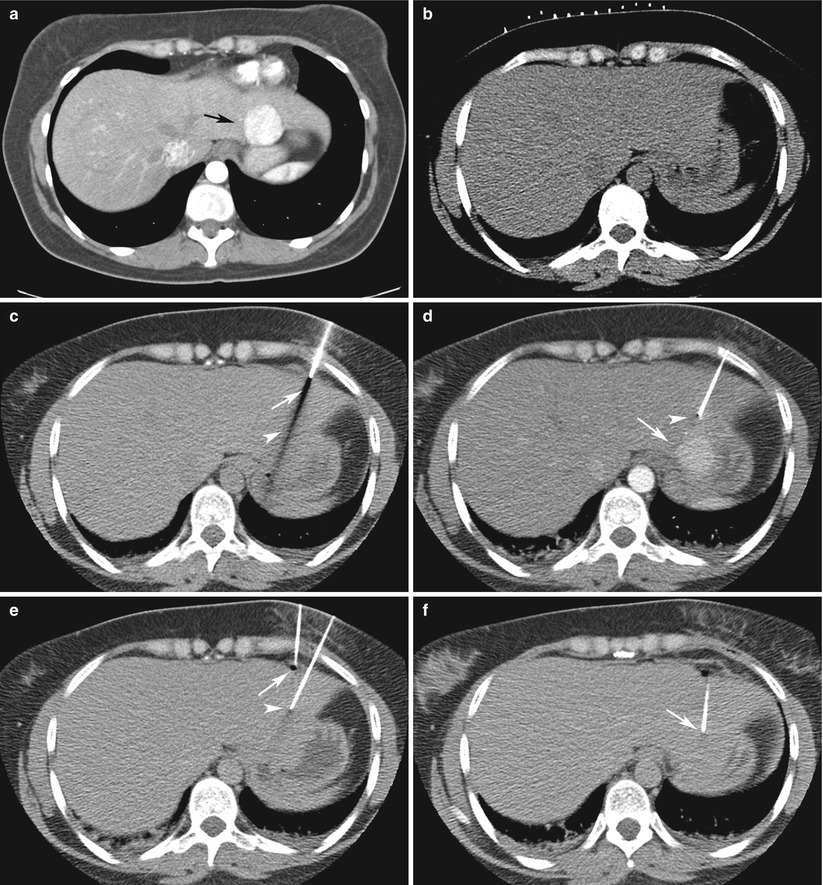

Fig. 13.3
CT-guided focal liver biopsy in a 54-year-old woman. Intravenous contrast-enhanced CT scan (a) performed to evaluate abdominal pain demonstrated multiple hypodense hepatic masses (arrowheads). Review of this scan at liver biopsy was necessary to plan grid placement (b, arrow). The hypodense masses (b, arrowheads) are visible on the non-contrast-enhanced preliminary scan. (c) A 17-gauge introducer needle (arrow) is advanced into the lesion. Subsequently, fine needle aspiration and core biopsy were performed using coaxial technique (not shown); pathology demonstrated metastatic small cell lung cancer

Fig. 13.4
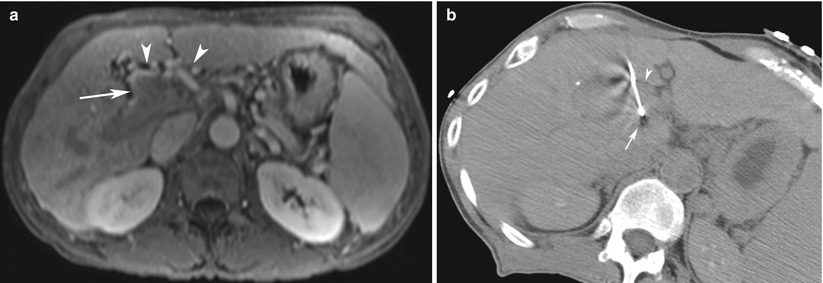
CT-guided focal liver biopsy in a 33-year-old woman with history of melanoma. (a) Intravenous contrast-enhanced CT scan of the abdomen and pelvis demonstrates a 3.5-cm arterially enhancing mass (arrow) in segment 2 of the liver. (b) Preliminary CT performed at liver biopsy demonstrates the lesion is isodense to normal unenhanced liver parenchyma. (c) A 17-gauge introducer needle (arrow) is advanced into segment 2 based on anatomic landmarks. Needle artifact (arrowhead) is prominent as the stylet is present within the needle. (d) Contrast-enhanced scan at biopsy demonstrates that the trajectory of the needle is not optimally aligned with the mass (arrow). Note, with the stylet removed, needle artifact is absent (arrowhead). (e) A second 17-gauge introducer needle (arrow) is advanced into the liver, using the first needle as a reference (arrowhead). (f) The second needle (arrow) is optimally aligned for biopsy of the mass. Pathology demonstrated focal nodular hyperplasia
Although many practitioners use a stepwise approach as outlined above, CT fluoroscopy (CTF) may be useful as it may reduce procedure duration [19, 20]. Although reduction in needle placement time has been documented, CTF has not proven to provide a statistically significant increase in procedure success [19]. In CTF, images may be reconstructed at a rate of up to 8 images per second and may be utilized in either continuous fluoroscopy mode or as a quick-check technique [19, 21]. With the former, the use of CT is analogous to conventional fluoroscopy, as needle manipulation is visualized in real time. The quick-check technique involves the use of single CT fluoroscopic spot images to intermittently check needle position. The quick-check technique is analogous to the conventional step-by-step CT technique but improves upon it in that the operator has use of a “floating table,” enabling quick movement of the table and the aid of a laser light to demarcate the axial slice, as well as hand and foot controls. Although the quick-check technique does not fully utilize the real-time capability of CTF, it improves on procedure time compared to conventional CT technique and significantly lowers radiation to patient and operator compared with the continuous fluoroscopy technique [19].
The success of any image-guided procedure is predicated on visibility of the lesion. CT-guided biopsy may be challenging when the targeted lesion is isodense to surrounding liver parenchyma on non-contrast images. In such cases, prior cross-sectional imaging that best demonstrates the lesion should be reviewed and the position of the lesion on preliminary CT images located based on anatomic landmarks. A reference needle (e.g., 20-gauge Chiba) may be advanced to this position using CT guidance (Fig. 13.4). Contrast-enhanced images can then be obtained and the location of the lesion relative to the reference needle assessed. A second needle entry site may be selected and the biopsy needle advanced to the lesion; although the lesion may no longer be enhancing while the second needle is inserted, the first needle continues to serve as a reference. Lesions identified on MRI images are occasionally not visible on US and contrast-enhanced CT. If MR guidance is unavailable, then CT guidance may be considered with the sole use of anatomic landmarks.
Another limitation of CT guidance is the lack of multiplanar capability. Hepatic dome lesions, for instance, may be better suited for US guidance if visible sonographically or MRI guidance, if available. If CT is to be used, gantry angulation may be helpful to avoid pleural or pulmonary transgression, although this may be time-consuming. A transpleural or transpulmonary trajectory may be unavoidable (22). The need for such a trajectory may be anticipated on prior imaging, in which case informed consent of the patient may include a discussion of pneumothorax and chest tube placement. The decision to pursue a transpleural or transpulmonary route depends on the clinical scenario; it may be performed safely although the operator should be prepared for a pneumothorax if one is detected on CT images (Fig. 13.5). A transpleural or transpulmonary route may be a relative contraindication in patients with tenuous clinical status who may not be able to tolerate a pneumothorax with chest tube placement. The risk of pneumothorax increases with the number of pleural transgressions; hence, the risk of pneumothorax with a transpulmonary approach is generally considered higher than with a transpleural approach as two pleural surfaces need to be traversed [22–25].
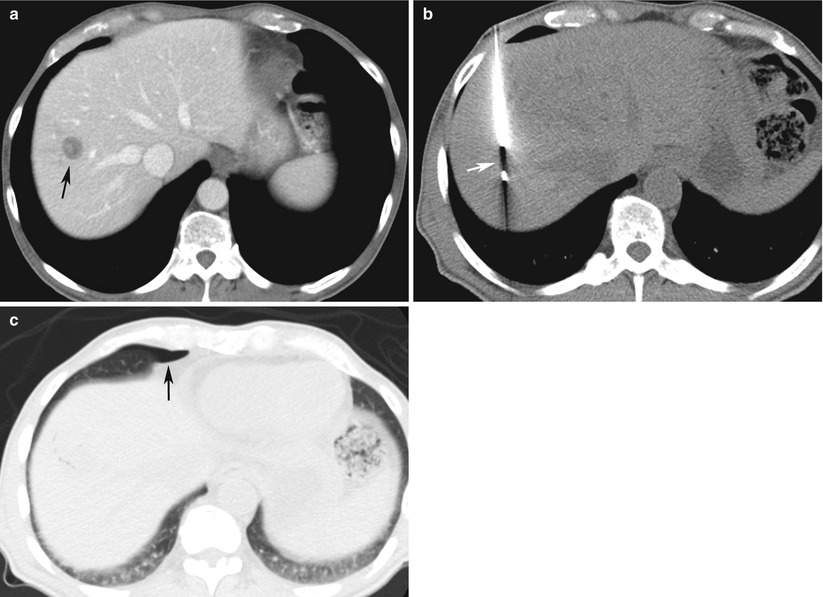

Fig. 13.5
Pneumothorax after CT-guided focal liver biopsy using a transpleural route in a 65-year-old man with history of pancreatic cancer. (a) Intravenous contrast-enhanced CT scan of the abdomen demonstrates a 1.8 cm hypodense mass (arrow) in segment 8 of the liver. (b) A core biopsy needle placed by coaxial technique encompasses the mass (arrow). (c) CT scan after removal of the needle demonstrates a small right anterior pneumothorax (arrow). The patient was asymptomatic and was discharged after a 2-h follow-up chest radiograph (not shown) demonstrated no pneumothorax. Pathology demonstrated metastatic pancreatic adenocarcinoma
MRI
MR guidance offers multiplanar imaging capability and excellent soft tissue contrast and lacks radiation [26]. However, its use is limited by procedure duration, cost, and limited availability compared to US and CT [27]. MR guidance is rarely indicated and may be helpful when encountering lesions within the hepatic dome, lesions which are poorly visible on US or CT, or when a nondiagnostic result is obtained after an US- or CT-guided biopsy.
The technique varies with the available equipment, and as such, a complete description of the available MR-guided techniques is beyond the scope of this chapter; the reader is referred to several excellent papers [26, 27]. In general, open- and closed-bore systems are available for interventional procedures. Open-bore designs offer improved patient access compared to closed-bore systems. C-shaped open-bore designs enable improved horizontal but poor vertical access; hence, some patients may need to be positioned in a decubitus position for anterior or posterior approaches [21]. Double-doughnut configuration open-bore systems enable excellent vertical and horizontal access [21]. These systems typically operate in the 0.2-T–0.5-T range, and hence, the quality of imaging is poorer compared to closed-bore systems that operate in the 1-T–1.5-T range but nevertheless may be sufficient for biopsy [26, 28]. Although a closed-bore design may offer better signal-to-noise and contrast-to-noise ratio, patient access is restricted, particularly in obese patients; instrument manipulations are thus often performed with the patient outside of the magnet as with conventional CT guidance. However, the availability of closed-, short-, wide-bore 1.5-T systems may offer improved access for interventions allowing needle manipulations when the patient is in the magnet while maintaining excellent imaging quality [27].
A step-by-step technique similar to CT has been described with both systems, although MR fluoroscopy may also be performed [27]. In general, preliminary non-contrast MRI images are obtained with a three-plane localizer sequence as well as fast T2-weighted spin-echo sequences; gradient-echo sequences can also be employed before and after gadolinium administration [26]. If a step-by-step technique is employed, as is commonly with conventional closed-bore systems, an MR-compatible grid visible on either T1- or T2-weighted sequences is applied to the skin. The technique is similar to CT, with the exception that the needle trajectory is not restricted to the axial plane [26]. T1- or T2-weighted sequences are obtained depending on the type of grid used. Software specific to the MR scanner is used to select the needle entry site, plan the angle of the needle trajectory, and measure the distance to the lesion. The needle is inserted at the entry site and is advanced using a stepwise technique, with each manipulation performed while the patient is outside the magnet.
Open-bore as well as closed-, short-, wide-bore systems may enable real-time guidance. By repeating fast imaging sequences, the operator’s palpating finger rather than a grid may be use to mark the needle entry site [26, 27]. Confirmatory images in two orthogonal planes may be obtained when the needle tip is at the edge of the lesion to detect any significant deviation of the needle [27]. If there is difficulty correcting needle deviation after several attempts using MR fluoroscopy (e.g., firm liver due to cirrhosis that restricts manipulation of soft MR-compatible needles), the stylet can be inserted into the needle while the patient is outside the magnet and the needle redirected, with intermittent imaging used as in a conventional step-by-step approach [27].
In general, the needle appears hypointense with a hyperintense rim on MR [26]. Many variables affect the degree of artifact induced by the needle. Distortion is worse with smaller needles, increased needle angle relative to B0, higher-field MR scanners, and gradient-echo versus spin-echo sequences [26].
Devices
Cytologists and pathologists may require multiple biopsy specimens in order to make an accurate and confident diagnosis. Biopsy techniques have evolved to facilitate obtaining multiple specimens while reducing the risk of complication. The most established method is the coaxial technique [21], although tandem- and single-needle techniques may also be performed.
The coaxial technique enables multiple tissue samples to be obtained with a single pass through overlying tissues and liver capsule, reducing patient discomfort, procedure duration, number of images required, and risk of complication [21]. This is of particular benefit in deep-seated lesions, in which single- or tandem-needle technique may be cumbersome [21]. In general, an introducer needle is advanced into the lesion under imaging guidance. Introducer needles are available from 11 G to 19 G, depending on the biopsy system to be used, although 17 G to 19 G is usually sufficient. Once the introducer needle is within the lesion, core biopsy and/or fine needle aspiration may be performed through the indwelling introducer needle.
Fine needle aspirates may be obtained with the use of a 20-G to 22-G hollow (e.g., Chiba) needle. The aspiration needle is advanced through the introducer needle. A “to-and-fro” motion is performed such that cells are acquired by capillary action. A 10-mL syringe may also be attached to the needle with a small degree of aspiration (1–3 mL) applied to improve cytologic yield, although suction is not applied while withdrawing the needle to reduce the amount of blood within the specimen.
Core biopsy guns are available in multiple designs and sizes. It is important to be familiarized with operation of the biopsy system available prior to use. The most commonly used is an automated, spring-activated side-cutting biopsy gun, available from 14 to 20 G. Automated devices generally provide more diagnostic specimens than their manual counterparts [21, 29, 30]. For hepatic lesions, 18-G to 20-G systems are most commonly used. Use of larger systems may be associated with a higher complication rate, although randomized trials are lacking [21, 31–33]. The core biopsy gun is advanced through the introducer needle while the inner trochar is retracted into the gun’s outer cannula. Subsequently, the inner trochar may be advanced into the lesion prior to firing the system; if desired, this allows the use of imaging to confirm that the side-cutting portion of the needle is well seated within the lesion prior to firing the system (Figs. 13.5 and 13.6). Typically, two or three 2-cm non-fragmented samples are sufficient for histopathology, although additional specimens may be needed if fragmented specimens are obtained. Representative methods of specimen preparation are summarized in Table 13.4.