Liver Malignancies
Kristy K. Brock
Laura A. Dawson
Liver metastases from colorectal cancer and primary liver cancer combined are a top source of global cancer morbidity and mortality. Local therapies can lead to sustained tumor control and cure when liver cancers are not diffuse or extrahepatic, with 5-year survival rates of 30% to 60% following resection. Unfortunately, many patients with primary and metastatic liver cancer are not eligible for surgery or other available local therapies. Radiation therapy (RT) is becoming established in the treatment of liver cancer, either on its own or combined with other local, regional, or systemic therapies.1
Challenges to the routine use of RT in this setting include the low whole liver tolerance to RT and the proximity of liver tumors to other dose-limiting normal tissues including the stomach and bowel. Thus, RT needs to be delivered conformally around liver tumors, sparing enough liver volume and maintaining all normal tissue tolerances to RT. Another challenge in delivering conformal RT to liver cancers is that the liver moves with breathing, and its mean position changes relative to the vertebral bodies from day to day (often referred to as “baseline shifts”).
Advances in imaging at the time of RT planning and delivery have made it possible for RT to be used to treat liver cancers safely, despite the challenges. Outcomes following RT for liver cancers vary tremendously, due in part to the variable patient selection but also perhaps due to the wide spectrum of RT doses, fractionations, and techniques used. Despite this, there is an increasing experience in the use of RT for liver cancers, with some reports showing long-term control following conformal RT.1 The chance of sustained local control is increased if higher doses of RT can be delivered. Tumoricidal doses of RT are most safely used if RT can be delivered with high precision and accuracy. The topic of this chapter is image-guided RT (IGRT) (including both imaging at the time of radiation planning and delivery), which facilitates improved precision and accuracy of liver cancer RT.
IMAGING AT SIMULATION
Because the image guidance strategy used at the time of RT delivery needs to consider liver motion due to breathing and the treatment planning strategy, imaging used for RT planning and breathing motion management will be described, in addition to the image guidance strategies used at the time of treatment delivery.
SIMULATION
The first step in treatment planning is to obtain a high-quality computed tomography (CT) image of the patient in the treatment position. The patient should be positioned in an immobilization device that will be used throughout the treatment process. The planning CT image acquisition parameters, including image resolution and contrast, must be optimized to demonstrate the tumor and normal tissues of interest. The optimal phase of contrast for tumor enhancement depends on the tumor type (i.e., primary or metastatic liver cancer). Image resolution must balance covering the necessary region of interest and dose. It must also address breathing motion. Eliminating breathing motion is essential for accurate tumor and normal tissue definition and to reduce any systematic error between simulation and treatment delivery. Two methods are commonly used to consider motion at the time of planning: (a) obtaining the image at breath-hold or (b) obtaining a respiration-correlated image set (i.e., four-dimensional [4D] CT).
DEFINING TREATMENT VOLUMES
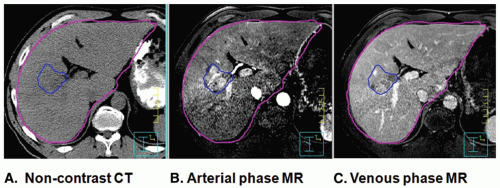
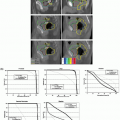
Once the simulation images have been obtained, the treatment planning volumes must be defined, as well as delineation of critical normal structures. Per the International Commission on Radiation Units (ICRU) 62 recommendations, the gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) need to be defined. The GTV is typically defined using an intravenous (IV) contrast-enhanced CT image obtained at breath-hold. The optimal phase depends on the disease type, with an arterial delayed image (20 to 30 seconds) typically best for hepatocellular carcinomas and a venous delay (50 to 70 seconds) typically best for liver metastases. A magnetic resonance image (MRI), with or without contrast, may also be used to aid in GTV definition in addition to the contrast-enhanced CT image.2 MRI is essential for GTV definition in patients with contraindications to IV CT contrast. Figure 13.1 displays a noncontrast CT, an arterial phase MRI, and a venous phase MRI of a patient with hepatocellular carcinoma. Fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging can also aid in identifying the GTV or extrahepatic metastases for patients with colorectal cancer liver metastases. FDG-PET is less useful for hepatocellular carcinoma because these cancers are FDG avid in only 50% of cases.
The CTV expansion for potential microscopic disease depends on the tumor type and varies across treatment centers and protocols, typically ranging from a 0 to 10 mm of isotropic expansion around the GTV within the liver. Biliary cancers are likely to have microscopic disease track the biliary trees, and a CTV margin of at least 10 mm is recommended. For many potent stereotactic body RT protocols for liver metastases, a CTV margin of 0 mm is recommended. Radiology-pathologic comparisons are required to help determine what CTV margin is most appropriate for each liver tumor type.
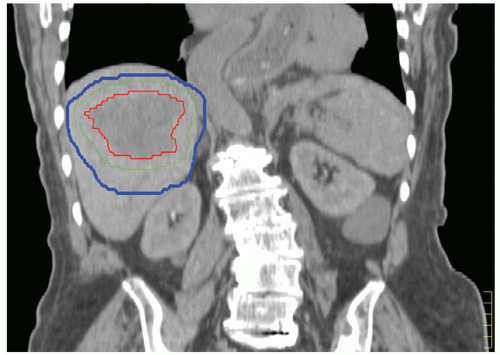
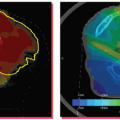
Patient-specific PTV expansions can help to ensure that the prescription dose is delivered to the target without the excessive normal tissue irradiation that would be required if PTV margins were based on population motion data. Breathing motion and the setup uncertainty expected at treatment delivery need to be considered in determination of the PTV. The breathing motion component of the PTV should be quantified by measuring the respiratory motion (methods of quantifying this motion are described later in this chapter) for patients treated while free breathing. ICRU 62 defines the internal target volume (ITV), which accounts for all internal uncertainties (e.g., breathing motion), thereby separating this uncertainty from the PTV required for setup error and other uncertainties. For patients treated in breath-hold or gating, the reproducibility and accuracy of the breath-hold or gating technique used for treatment must be included in this PTV margin. Acquiring a breath-hold image for CT simulation allows the breathing margin associated with the PTV margin to be applied asymmetrically (i.e., if an exhale breath-hold image is acquired for treatment planning, the breathing component of the PTV margin can be applied in the inferior and anterior directions with no specific breathing PTV margin in the superior direction or posterior direction).3 In addition to the PTV margin required for breathing motion (i.e., the ITV), an additional PTV margin for setup error, changes in position of the soft tissues relative to the bones from day to day (if no image guidance is used), and accuracy of the image guidance approach (if image guidance is to be used) need to be considered. Figure 13.2 displays a CT image obtained at end exhale with an anisotropic PTV margin applied based on the patient’s breathing motion—15 mm inferiorly to account for breathing motion and an additional 5-mm setup uncertainty PTV margin in all directions to account for residual setup error.
INTEGRATION OF MULTIMODALITY IMAGING
The use of multimodality imaging (e.g., MRI or PET in addition to CT) for tumor definition can improve the accuracy of the treatment planning process. MRI is particularly useful in target definition in patients with allergies or contraindications to IV CT contrast. Although FDG-PET does not add anatomic or staging information in most primary liver cancers, it is used more commonly to help stage and define targets in metastatic colorectal cancer.4,5 However, PET scans are obtained
over many breathing cycles, and strategies to account for breathing motion are not routinely available. A nonrespiratory sorted PET scan results in blurred images and blurred tumors. It is important to note that due to asymmetric breathing patterns, which are typical for most patients,6,7 this blurring may lead to a systematic error in target definition if the PET image is used alone without considering CT or MRI. Respiration-correlated PET is under investigation but is currently not available clinically.8,9
over many breathing cycles, and strategies to account for breathing motion are not routinely available. A nonrespiratory sorted PET scan results in blurred images and blurred tumors. It is important to note that due to asymmetric breathing patterns, which are typical for most patients,6,7 this blurring may lead to a systematic error in target definition if the PET image is used alone without considering CT or MRI. Respiration-correlated PET is under investigation but is currently not available clinically.8,9
The integration of all imaging requires image registration. Image registration is likely to be more successful and efficient if measures are taken to reproduce the patient geometry between each imaging modality, such as using the same immobilization device and obtaining the images at the same breath-hold position. Although soft tissue deformation is likely to exist between imaging modalities, at least to some degree, deformable image registration is not currently available in commercial treatment planning systems. Rigid image registration can be optimized in the presence of deformation by focusing the registration on the specific region of interest. For example, if MRI and CT are to be fused to help in tumor definition, the rigid registration should focus on the liver, and if liver deformation is substantial, the rigid registration should focus on the region of the liver containing the tumor. If a substantial amount of deformation is observed between the liver on each imaging modality, additional PTV margins may be necessary to account for residual uncertainties due to deformation, especially if the secondary image is the only image used for tumor definition (e.g., if there is a contraindication for CT IV contrast and the CT is used for treatment planning).
MEASURING MOTION
Ideally, the patient-specific breathing motion would be known and considered in PTV margins because studies have shown a large interpatient variation in respiratory motion. The superior-inferior direction tends to exhibit the largest motion, ranging from 5 to 40 mm.10 Motion of either the liver tumor itself or a surrogate, such as the diaphragm or inserted fiducial markers, can be measured using several methods, including 4D CT, repeat breath-hold images at two or more states, kilovoltage (kV) fluoroscopy, cine MRI, or respiratory-correlated volumetric imaging acquired at the treatment unit.
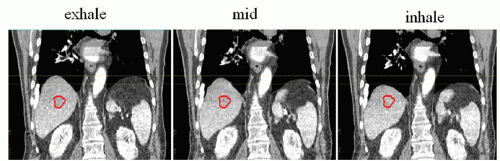
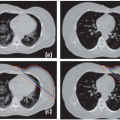
4D CT provides a multiphase three-dimensional (3D) image set to measure motion in all directions as well as hysteresis (i.e., differences in the inhale to exhale and exhale to inhale tumor trajectory).11,12 However, using 4D CT for calculating tumor-specific motion may be difficult because tumors in the liver are difficult (and often impossible) to see without the use of IV contrast. The use of IV contrast during 4D CT is currently being investigated; however, the short window for the optimum tumor contrast (15 seconds) and the longer scan times associated with 4D CT (approximately 100 seconds) mean that the image quality and the ability to accurately delineate the tumor edges for all phases of the CT may be reduced compared to a breath-hold triphasic contrast CT. Nonetheless, the feasibility of this approach has been described,13 and combined with diagnostic quality imaging, contrast 4D CT provides more information about the tumor motion itself, which may vary from the overall average liver motion. Figure 13.3 shows three example phases (exhale, inhale, and mid-ventilation) of a 4D CT scan. The tumor, which was delineated on the exhale reconstruction, is overlaid onto the mid-ventilation and inhale reconstruction for reference. 4D CT images can also be combined together to form a maximum intensity projection (MIP) to aid in contouring the ITV.14,15 This is more commonly used in the lung, where the difference in intensity between the lung and the tumor may be substantial.
Repeat breath-hold images at the end exhale and normal inhale position also provide 3D image sets that allow motion to be measured in all directions; however, without the intermediate phase reconstructions, hysteresis cannot be detected. Repeat CT images may also lack significant tumor contrast in the second image, and voluntary breath-holds may overestimate motion during quiet breathing. Care must be taken to properly coach the patient to hold their breath at a normal, not deep, inspiration or exhalation breath-hold when repeat breath-holds are used for simulation.
Fluoroscopy provides real-time imaging of the diaphragm and lung interface, which can be used as a surrogate measure of liver tumor motion in the superior-inferior direction.3 Left-right motion, however, is not discernable. Anterior-posterior motion may be detected using lateral fluoroscopy; however, this is not commonly used. Fiducial markers close to the tumor can provide better estimates of liver tumor motion compared to the diaphragm and can facilitate assessment of anterior-posterior and left-right tumor motion.16
MRI can provide tumor visualization even in the absence of contrast, and thus, MRI can be used to improve the temporal sampling of breathing motion compared with 4D CT. Cine
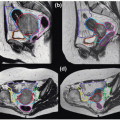
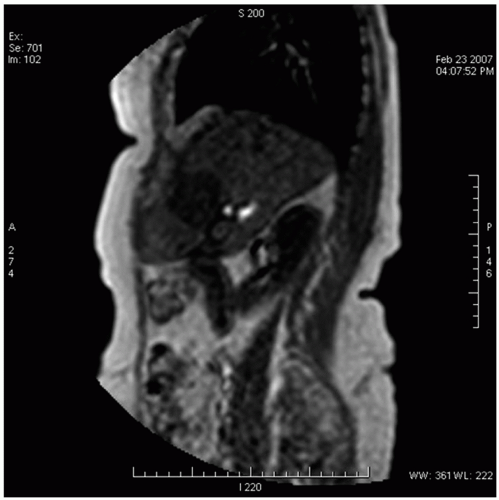
MRI can also be used to measure tumor-specific motion using two-dimensional (2D) temporal images in multiple planes (e.g., coronal and sagittal)17,18 or volumetric imaging (which is not widely available). The frequency of MRI acquisition ranges from one to three images per second, and acquisition length should be long enough to estimate variability in breathing and the extremes of motion. Because the 3D motion is measured on 2D images, out-of-plane motion can compromise the measurement of the true motion of the tumor. Research is pursuing the use of 3D volumetric cine MRI.19 Figure 13.4 illustrates a 2D slice from a 3D volumetric cine image obtained using a multishot electronic portal imaging sequence with a four-element SENSE body coil on a Philips Intera 1.5-T MRI scanner (Philips, Andover, Mass). A series of 60 consecutive 3D volume images of 45 slices were acquired in 1.2 seconds each, at 3.5 × 3.5 mm resolution in plane and 4.5 mm slice thickness.
MRI can also be used to measure tumor-specific motion using two-dimensional (2D) temporal images in multiple planes (e.g., coronal and sagittal)17,18 or volumetric imaging (which is not widely available). The frequency of MRI acquisition ranges from one to three images per second, and acquisition length should be long enough to estimate variability in breathing and the extremes of motion. Because the 3D motion is measured on 2D images, out-of-plane motion can compromise the measurement of the true motion of the tumor. Research is pursuing the use of 3D volumetric cine MRI.19 Figure 13.4 illustrates a 2D slice from a 3D volumetric cine image obtained using a multishot electronic portal imaging sequence with a four-element SENSE body coil on a Philips Intera 1.5-T MRI scanner (Philips, Andover, Mass). A series of 60 consecutive 3D volume images of 45 slices were acquired in 1.2 seconds each, at 3.5 × 3.5 mm resolution in plane and 4.5 mm slice thickness.
 Get Clinical Tree app for offline access 
|