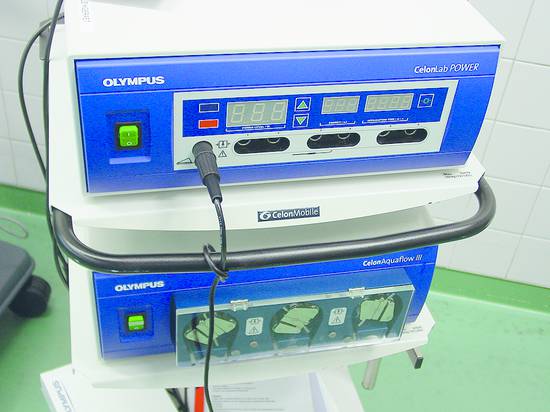
Local Ablative Procedures for Liver Tumors, Radiofrequency Ablation Local ablative procedures for the treatment of liver tumors are classified as follows: Percutaneous injection of ethanol, acetic acid, or other agents Heat-based techniques Radiofrequency thermoablation, RFA Microwave ablation Laser-induced thermotherapy (LITT) High-intensity focused ultrasound (HIFU) Cryoablation (generally done surgically due to the larger diameter of the probes)1–3 RFA is the most widely used of the above modalities and will be the focus of this chapter. RFA can be performed percutaneously under general anesthesia with endotracheal intubation or under local anesthesia plus sedation. The placement of the applicator and the progress of treatment are monitored by ultrasound, CT, or MRI. The choice of imaging modality depends on availability and tumor visibility. RFA can also be performed in the setting of an open or laparoscopic operation under general anesthesia and with intraoperative ultrasound guidance. The local ablation of liver tumors is usually done with curative intent. This applies mainly to small hepatocellular carcinomas (HCC) up to 5 cm in size as well as colorectal tumors metastatic to the liver, also up to 5 cm (4 cm) in size (details below). The decision for local ablation over resection should take into account patient age, comorbidity, parenchymal reserve, and the tumor distribution in the liver weighed against the invasiveness of the procedure. Palliative reduction of tumor mass is done for metastatic neuroendocrine tumors, for example, and is repeatedly tried on other tumor entities such as liver metastases of breast cancer. Thus, multimodal palliative local ablation is routinely employed even in patients with predominant liver metastases.4–6 On the other hand, the principle of tumor mass reduction has been generally acknowledged and specified in guidelines only for certain tumor entities, e.g., for symptom control in neuroendocrine cancers and for symptom relief (pain and pressure) in carcinoid liver metastases.7–14 Local ablative treatments can be effectively combined with other procedures for both curative and palliative intent. The combination of surgical resection and ablative treatment for localized colorectal metastases with an unfavorable bilobular distribution is practiced at many centers with curative intent. The treatment options for hepatocellular carcinoma in a cirrhotic liver are liver transplantation, partial hepatectomy, local ablative therapies, transarterial chemoembolization (TACE), radioembolization (selective internal radiation therapy [SIRT], etc.) and, in advanced stages, chemotherapy with sorafenib (Nexavar, Bayer HealthCare Pharmaceuticals). The favorable results of percutaneous ethanol injection for HCC are attributable to the hard perifocal reaction (hepatic cirrhosis), encapsulation, and decreased portal venous blood flow causing less washout of the ethanol. However, several recent studies comparing PEI and RFA have shown that RFA yields better long-term results and requires fewer treatment sessions.15–17 As a result, PEI has been replaced by RFA at most European centers. The 5-year survival rates following RFA in a selected cohort with small, well-differentiated HCC are as high as 80% in our patients, although the rates in most cases were <45%.1,2 Two controlled, randomized studies have shown that the RFA of solitary HCCs up to 5 cm in size, or as many as three HCCs up to 3 cm in size, is comparable to surgical resection in terms of survival rates at 3 and 4 years while having a lower procedure-related mortality.18,19 Given the significantly lower costs and invasiveness of ablation, it may be considered superior to resection in patients with localized HCC. This is particularly true in patients with preexisting portal hypertension, which is a relative contraindication to surgery. A particular advantage of ablation is that it is a parenchyma-sparing procedure. This is of major benefit in HCC patients, most of whom have a cirrhotic liver. New multipolar ablative techniques involving the simultaneous use of several ablation probes (see below) can achieve reasonably good tumor control rates of 85% even with large HCCs up to 9 cm in diameter.20 Treatment concepts and protocols for hepatocellular carcinoma have been published by numerous authors and summarized, for example, in the stage-oriented treatment recommendations of the Barcelona Clinic Liver Cancer (BCLC) staging system and other systems.21,22 The literature may be consulted for details on the differences between recommendations.23–25 In patients with colorectal metastases confined to the liver, liver resection according to S3 guidelines is indicated as a potentially curative treatment whenever an R0 resection of all metastases is technically feasible.26,27 The 5-year survival rates after partial hepatectomy range from 25 to 45%. Approximately 70% of patients will develop a recurrence (usually intrahepatic) after the resection.26 Local ablative procedures with curative intent have played a growing role in the management of colorectal liver metastases in recent years. RFA may be performed alone or may be combined with resection, depending on the size of the lesions. Several cohort studies in recent years, some involving several hundred patients, have shown that RFA can not only achieve permanent local devitalization of liver metastases but can also provide 5-year survival rates of 24 to 43%.28–30 These results are comparable to those of partial hepatectomy. This is all the more remarkable when we consider that RFA patients generally have a poorer long-term prognosis, due in part to their higher comorbidity than candidates for resection. On the other hand, the rate of intralesional recurrence, which is approximately 15% even in experienced hands using current aggressive ablative techniques (see below), is higher than the rate of surgical margin recurrence after resection (<5–10%). But given the fact that approximately 70% of patients will develop new metastases anyway, the higher intralesional recurrence rate may have no more than a minor impact on long-term survival. On the critical side, it should be noted that no controlled, randomized data are available on the comparison of resection and local ablative procedures. This is also reflected in current guidelines, which unfortunately do not offer any treatment recommendations.26,27,31,32 At the same time, no controlled, randomized data have yet been published on the resection of liver metastases, which is nevertheless considered the gold standard. Practice Ultrasound-guided interventional procedures should be integrated into an overall oncologic concept, and interdisciplinary oncologic consultation is essential. The combination of minimally invasive interventional procedures with chemotherapeutic treatment strategies should be considered. Ultrasonography has established itself as the first-line imaging modality for local ablative procedures on the liver (except for LITT33 and intraoperative cryoablation). Contrast-enhanced ultrasound is used after the intervention to evaluate response. Prerequisites are that the lesions must be accessible and clearly visible by ultrasound. CT (or MRI) is the preferred imaging modality in all cases where ultrasound guidance is not possible or is subject to significant limitations. Local expertise and personal experience should be considered in determining the modality of choice. In all cases, contrast-enhanced imaging should be used during the intervention to confirm effective treatment. The advantages of ultrasonography are its real-time capability, making it easier to control than the static modalities of CT and MRI, its high spatial resolution, and its availability. Ultrasound has proven particularly advantageous in the puncture of subdiaphragmatic liver lesions (in which the needle is angled cephalad), as it can safely avoid accidental puncture of the pleura and lung. Ultrasound-guided tissue ablation (e.g., of liver tumors and renal neoplasms) is technically feasible in all cases where ultrasound can define a safe access route to the lesion. The number of tumors, their locations, and preferably their histology (grade) should be known prior to treatment so that complete tumor ablation can be accomplished in one or more steps. The maximum number of tumors that can reasonably be ablated is not clearly defined but ranges from 3 to 5 at most centers. Again, there is no established standard for the maximum tumor size that can be ablated. The maximum size of ablatable tumors is generally in the range of 4 to 5 cm. New techniques such as the simultaneous or consecutive use of multiple ablation probes and stereotactically guided RFA can successfully treat tumors up to 10 cm in diameter.20,34,35 Because a single ablation needle can produce necrotic zones no larger than about 2 to 3 cm (depending on the application system), multineedle systems have been introduced that can produce zones as large as 7 cm with simultaneous use. Consecutive needle use can produce even larger ablation volumes. Tumors larger than 2 cm require multineedle ablation to destroy the tumor along with a safety margin of at least 5 to 10 mm.36,37 Attention must be given to vulnerable structures that border directly on the tumor or ablation zone. This particularly applies to the small intestine and colon, which are highly thermosensitive. Thermally induced bowel perforation is a feared complication of RFA. The stomach, gallbladder, and diaphragm are less thermosensitive, and tumors in proximity to these structures can generally be ablated without difficulty. The ultrasound-guided intraperitoneal injection of glucose solution to separate hollow organs from the liver surface before ablation is an established method of avoiding thermal injury (saline solution is contraindicated due to its electrical conductivity). The RFA of lesions that border directly on central bile ducts is contraindicated due to the risk of thermal injury. Close proximity to large hepatic vessels is also problematic because these vessels are efficient heat eliminators, potentially leading to incomplete ablation or local recurrence. This problem can be solved by the concomitant, transient embolization of arterial branches supplying the liver, causing a temporary reduction in blood flow.38 Nevertheless these procedures lack a stable evidence base. The contraindications to local ablation are the same as the general contraindications to interventional procedures based on the risk and benefit for the individual patient, giving particular attention to the age, comorbidity, and desires of the patient. Acetylsalicylic acid use is no more than a relative contraindication. Clopidogrel should be discontinued before the intervention if at all possible. The use of monopolar systems is contraindicated by cardiac pacemakers and implanted defibrillators, which must be turned off prior to ablation. Bipolar systems are safe to use in patients with active pacemakers. Status post biliary–enteric anastomosis is a relative contraindication, as this would predispose to abscess formation in the zone of thermal necrosis. If it is decided to proceed with RFA regardless of the anastomosis, antibiotic prophylaxis should be given (see below). Contraindications relating to tumor location were discussed in the previous section. Antibiotic prophylaxis with a third-generation cephalosporin (e.g., ceftriaxone, 2 g IV) or agents with a comparable spectrum can be given as a (single) bolus 30 to 60 minutes before the intervention. While this has been recommended by some authors, it is not a widely adopted practice. Prophylaxis is mandatory in patients who have had a previous biliary–enteric anastomosis or papillotomy (see above). There is no consensus as to whether RFA should be performed under sedation, sedation/analgesia, or general anesthesia with endotracheal intubation. Nevertheless, many centers have come to favor general anesthesia, especially when treating larger tumors with multiple probes and long ablation times, because these interventions are very painful. Advantages of general anesthesia are greater patient comfort and better control of the intervention, including significantly better control of respiration during needle insertions. One disadvantage is the higher logistical cost. Local anesthesia and sedation/analgesia follow the principles described elsewhere in this book (Chapter ▶ 11). Treatment planning takes into account the size (diameter) and location of the tumor (liver segment; adjacent vessels; at-risk structures such as bowel, bile ducts, or heart), the proposed access route, and the target position of the electrodes. Preinterventional CT or MR images are usually available and should be consulted to assist planning. The patient should be placed in an optimum position; left lateral decubitus is often advantageous for lesions in the hepatic right lobe. Key considerations are the number, type, and (recommended) spacing of the electrodes, power settings, and energy levels (taking into account dosimetry tables). In ultrasound-guided RFA using multiple probes, it should be noted that the placement of the ultrasound transducer for inserting additional probes may be hampered by probes that have already been placed. This should be anticipated at the planning stage and avoided by suitable placement strategies. The ablation should be planned and carried out to destroy all the tumors and in addition >5 mm (preferably >10 mm) of normal liver parenchyma on all sides. This is necessary to avoid high rates of intralesional recurrence.37 Accurate planning and positioning of the ablation needles is the basis for successful RFA. The characteristics of standard ablation systems available on the market are listed in ▶ Table 19.1. An RF generator (from Celon/Olympus) is illustrated in ▶ Fig. 19.1. The recommended instrument setup for RFA is shown in ▶ Fig. 19.2. Fig. 19.1 RF generator (Celon/Olympus). Fig. 19.2 The materials necessary for RFA are laid out on a sterile table (see text for details). The setup includes sterile sponges, draping material, sterile ultrasound gel, a transducer sheath and needle holder, and an RFA needle with irrigation system. The principle of RFA involves mechanisms in which energy at a frequency of, say, 350 kHz and a power of 4 to 40 W (up to 250 W in bipolar and multipolar systems) is converted to heat within the tissue. In this principle, the tissue forms part of a high-frequency, low-resistance electrical circuit that includes a grounding pad applied to the skin (▶ Fig. 19.3). Fig. 19.3 The treated tissue is part of a high-frequency, low-resistance electrical circuit that includes a grounding pad. This mechanism is like that of monopolar electrocoagulation (microarc-free “soft” coagulation), which has been applied surgically for many years. Heating occurs chiefly in the area where high-frequency fields and high-frequency currents are most concentrated. The energy transfer of high-frequency currents diminishes with the square of distance, while the heat generated at the targeted site increases linearly with the application time. In a favorable case, the high-frequency current produced by the power control unit flows through the pathologic tissue located at the distal end of the applicator, heating the tissue to temperatures up to 100°C. This is sufficient to cause coagulation (denaturing) and necrosis of the affected tissue. The size of the ablated tissue volume depends on the applicators used, the amount of energy applied, and local tissue anatomy. The coagulated necrotic tissue is subsequently broken down and converted to scar tissue by endogenous mechanisms in the body. In this way a tumor can be completely destroyed in properly selected cases. Monopolar systems employ a neutral electrode (grounding pad) that is applied to the body surface. The current flows between the ablation probe in the tumor and the neutral electrode, generating heat that is distributed concentrically around the probe.
19.1 Concepts (Curative, Palliative, Multimodal)
19.1.1 Hepatocellular Carcinoma
19.1.2 Colorectal Carcinoma
19.2 Selection of Imaging Modality (Ultrasound, CT, MRI)
19.3 Indications
19.3.1 Number of Tumors
19.3.2 Tumor Size
19.3.3 Tumor Location
19.4 Contraindications
19.5 Preparations
19.5.1 Antibiotic Prophylaxis
19.5.2 Local Anesthesia, Sedation, Sedation/Analgesia, and General Anesthesia
19.5.3 Treatment Planning
19.6 Materials
19.6.1 Standard Materials
Characteristic
Description
Needle type
Smooth needle
Multiple needles in one shaft
Retractable multiple-tined needles
Retractable coiled electrode
Mode of current application
Monopolar
Bipolar (one probe), smooth or retractable
Bipolar (between multiple probes)
Cooling
Not cooled
Liquid-cooled through two channels
Cluster-cooled
Surface
No openings
Multiple side holes for fluid outflow
Control
Impedance-controlled
Resistance-controlled
Temperature-controlled
Current delivery
Continuous
Dose alternating
Consecutive between the electrodes
Simultaneous to the electrodes
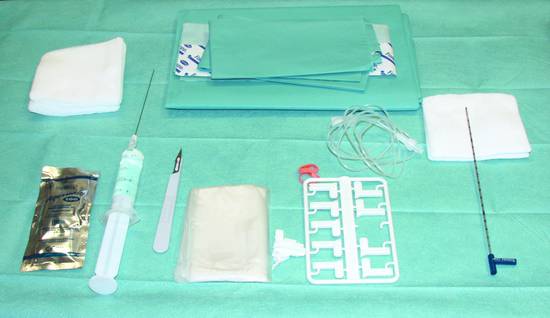
19.6.2 Basic Principle
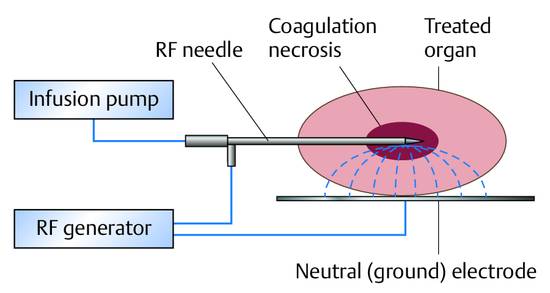
19.6.3 Monopolar versus Bipolar and Multipolar Systems
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree