Key points
- •
Luminal imaging techniques do not adequately evaluate extracranial carotid atherosclerotic plaque burden and characteristics in patients with low-grade carotid stenosis.
- •
Although multiple randomized controlled trials have not indicated an advantage to surgery over medical management in the low-grade stenosis population, there is an associated risk of stroke.
- •
Plaque features such as intraplaque hemorrhage, fibrous cap rupture, and ulceration, among others, confer an increased risk of stroke in low-grade stenosis.
- •
Considering that atherosclerosis is a systemic disease, vessel wall MR imaging can help determine the culprit lesion in the setting of cryptogenic stroke.
- •
Carotid vessel wall MR imaging can be helpful in identifying likelihood of plaque and its associated risk in other vascular beds that are not as easily imaged.
Introduction
Stroke is the second most common cause of mortality and a leading cause of morbidity worldwide. Approximately 80% of strokes are presumed to be ischemic in etiology, with 20% to 30% arising from extracranial carotid atherosclerosis. In the setting of extracranial carotid artery disease, the decision to treat symptomatic patients surgically or with carotid artery stenting has traditionally relied on the degree of luminal stenosis on catheter angiography based on the results of randomized controlled trials. Pooled data from the trials showed a 16% absolute reduced 5-year risk of future stroke events in patients with 70% or greater stenosis undergoing carotid endarterectomy in comparison with medical management.
Over the past 10 years, however, investigation has placed less emphasis on the degree of stenosis and more on plaque features that confer lesion vulnerability. These vulnerable plaque features support the hypothesis, as in coronary artery disease, that many cerebral infarctions result from plaque rupture and distal embolization or acute occlusion, and not long-standing hypoperfusion. Multiple studies have provided evidence that plaques resulting in moderate stenosis can rupture and result in acute ischemic events. Barnett and colleagues indicated a significantly increased 5-year risk of ipsilateral stroke ( P = .045) in patients with 50% to 69% stenosis treated medically (22.2%) compared with those treated surgically (15.7%). For those with less than 50% stenosis in the North American Symptomatic Carotid Endarterectomy Trial (NASCET), the stroke rate was lower in the surgical group relative to the medically managed group, although this did not reach statistical significance (14.9% vs 18.7%, P = .16). According to pooled data from the randomized control trials, the 5-year reduction in ipsilateral stroke rate was 4.6% for surgical patients with moderate (50%–69%) stenosis. There was no benefit in stroke rate for patients with 30% to 49% stenosis between the surgical and medical management groups, whereas there was increased risk of stroke in the surgical group for those with less than 30% stenosis (absolute risk reduction −2.2%, P = .05). The European Carotid Surgical Trial reported a 1.3% rate of ipsilateral ischemic stroke lasting longer than 7 days in patients with symptomatic mild (0%–29%) stenosis during a 3-year follow-up (0.43% per year). Fritz and Levien reported an 8.6% rate of ipsilateral ischemic events during a 2-year follow-up of 35 symptomatic patients with low-grade carotid stenosis or ulcerated plaque on medical management. In the setting of carotid atherosclerosis, including lesions resulting in low-grade carotid stenosis, there may be other lesions ipsilateral to the symptomatic side including aortic and intracranial plaques. Plaque-component characterization can provide important information to help stratify the likelihood that the carotid plaque is indeed the culprit lesion so that an appropriate treatment strategy can be implemented, including resection of the low-grade lesion. With moderate or severe carotid stenosis the associated plaques are presumed to be the culprits, and the randomized controlled trials have indicated the value of surgical intervention. The overestimation of stroke risk by contemporary standards in the medically managed groups for these trials that predate statin treatment suggests potential overtreatment of high-grade stenosis by surgery, and MR vessel wall imaging might also help to stratify high-grade lesions for a more appropriate balance of medical versus surgical treatments.
Although the rate of stroke in low-grade extracranial carotid stenosis differs based on the aforementioned trials, these trials have indicated that the benefit of surgery in low-grade stenosis may not improve the outcome over medical management when surgical risks are taken into consideration. However, these trials are based on narrowing to guide surgical management, which cannot stratify the risk of rupture for low-grade lesions and identify those at high enough risk to benefit from endarterectomy, and for this reason plaque characterization by MR imaging can potentially play an important role. Furthermore, despite a lower risk of stroke from low-grade carotid plaque compared with high-grade lesions, the chance for stroke from low-grade plaque cannot be discounted when considering the high prevalence of this disease. The occurrence of low-grade carotid stenosis in elderly populations is frequent, as 75% of men and 62% of women older than 64 years had carotid stenosis on ultrasonography in the Cardiovascular Health Study, whereas only 7% of men and 5% of women had stenosis greater than 49%.
Introduction
Stroke is the second most common cause of mortality and a leading cause of morbidity worldwide. Approximately 80% of strokes are presumed to be ischemic in etiology, with 20% to 30% arising from extracranial carotid atherosclerosis. In the setting of extracranial carotid artery disease, the decision to treat symptomatic patients surgically or with carotid artery stenting has traditionally relied on the degree of luminal stenosis on catheter angiography based on the results of randomized controlled trials. Pooled data from the trials showed a 16% absolute reduced 5-year risk of future stroke events in patients with 70% or greater stenosis undergoing carotid endarterectomy in comparison with medical management.
Over the past 10 years, however, investigation has placed less emphasis on the degree of stenosis and more on plaque features that confer lesion vulnerability. These vulnerable plaque features support the hypothesis, as in coronary artery disease, that many cerebral infarctions result from plaque rupture and distal embolization or acute occlusion, and not long-standing hypoperfusion. Multiple studies have provided evidence that plaques resulting in moderate stenosis can rupture and result in acute ischemic events. Barnett and colleagues indicated a significantly increased 5-year risk of ipsilateral stroke ( P = .045) in patients with 50% to 69% stenosis treated medically (22.2%) compared with those treated surgically (15.7%). For those with less than 50% stenosis in the North American Symptomatic Carotid Endarterectomy Trial (NASCET), the stroke rate was lower in the surgical group relative to the medically managed group, although this did not reach statistical significance (14.9% vs 18.7%, P = .16). According to pooled data from the randomized control trials, the 5-year reduction in ipsilateral stroke rate was 4.6% for surgical patients with moderate (50%–69%) stenosis. There was no benefit in stroke rate for patients with 30% to 49% stenosis between the surgical and medical management groups, whereas there was increased risk of stroke in the surgical group for those with less than 30% stenosis (absolute risk reduction −2.2%, P = .05). The European Carotid Surgical Trial reported a 1.3% rate of ipsilateral ischemic stroke lasting longer than 7 days in patients with symptomatic mild (0%–29%) stenosis during a 3-year follow-up (0.43% per year). Fritz and Levien reported an 8.6% rate of ipsilateral ischemic events during a 2-year follow-up of 35 symptomatic patients with low-grade carotid stenosis or ulcerated plaque on medical management. In the setting of carotid atherosclerosis, including lesions resulting in low-grade carotid stenosis, there may be other lesions ipsilateral to the symptomatic side including aortic and intracranial plaques. Plaque-component characterization can provide important information to help stratify the likelihood that the carotid plaque is indeed the culprit lesion so that an appropriate treatment strategy can be implemented, including resection of the low-grade lesion. With moderate or severe carotid stenosis the associated plaques are presumed to be the culprits, and the randomized controlled trials have indicated the value of surgical intervention. The overestimation of stroke risk by contemporary standards in the medically managed groups for these trials that predate statin treatment suggests potential overtreatment of high-grade stenosis by surgery, and MR vessel wall imaging might also help to stratify high-grade lesions for a more appropriate balance of medical versus surgical treatments.
Although the rate of stroke in low-grade extracranial carotid stenosis differs based on the aforementioned trials, these trials have indicated that the benefit of surgery in low-grade stenosis may not improve the outcome over medical management when surgical risks are taken into consideration. However, these trials are based on narrowing to guide surgical management, which cannot stratify the risk of rupture for low-grade lesions and identify those at high enough risk to benefit from endarterectomy, and for this reason plaque characterization by MR imaging can potentially play an important role. Furthermore, despite a lower risk of stroke from low-grade carotid plaque compared with high-grade lesions, the chance for stroke from low-grade plaque cannot be discounted when considering the high prevalence of this disease. The occurrence of low-grade carotid stenosis in elderly populations is frequent, as 75% of men and 62% of women older than 64 years had carotid stenosis on ultrasonography in the Cardiovascular Health Study, whereas only 7% of men and 5% of women had stenosis greater than 49%.
Modifications in medical management
Since the publication of the trials for evaluation of disease management based on luminal stenosis, there have been significant changes to the optimal medical management regimen that have modified stroke risk in medically managed patients. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), a class of cholesterol-lowering drugs, have become a staple of atherosclerosis-related stroke management. The Stroke Prevention by Aggressive Management in Cholesterol Levels Investigators (SPARCL) randomized 4731 patients with prior stroke or transient ischemic attack (TIA) (between 1 and 6 months from the event), no known coronary heart disease, and low-density lipoprotein cholesterol (LDL-C) between 100 and 190 mg/dL to 80 mg atorvastatin therapy or placebo. The 5-year absolute reduction in risk of major cardiovascular events was 3.5% (hazard ratio [HR] 0.8, 95% confidence interval [CI] 0.69–0.92; P = .002) with no significant difference in mortality rates. Of the 4731 randomized patients, 4278 were evaluated for carotid disease, and of those 1007 were found to have carotid stenosis. By randomization to the atorvastatin group, the incidence of any cardiovascular events was reduced by 42% relative to placebo (HR 0.58; 95% CI 0.46–0.73; P <.00001). The risk of cerebrovascular events (TIA or stroke) was reduced by 34% in the atorvastatin group (HR 0.66, 95% CI 0.5–0.89; P = .005). The risk of undergoing carotid revascularization was reduced by 54% (HR 0.44, 95% CI 0.24–0.79; P = .006). The Heart Protective Study Collaborative Group randomized 20,536 patients in the United Kingdom with coronary artery disease, other occlusive artery disease, or diabetes mellitus to 40 mg simvastatin or placebo groups, and found a significant reduction in fatal and nonfatal stroke risk in the statin group (4.3% vs 5.7%, P <.0001). For the first occurrence of major vascular events, there was a 24% reduction in the event rate (19.8% vs 25.2%, P <.0001). Hegland and colleagues evaluated 230 patients with 318 carotid arteries with at least 40% carotid stenosis on carotid ultrasonography without occlusion or referral for carotid revascularization. Of these, 171 were not receiving statin therapy while 147 were treated with simvastatin. There was no significant difference in baseline stenosis but there was a significant difference in change in stenosis, as the group that did not receive statin therapy had a +4.9% change in stenosis while the simvastatin group had on average a stenosis change of −10% ( P <.001). A meta-analysis that included more than 90,000 patients evaluated all trials testing the effect of statin drugs on the incidence of strokes and carotid intima-media (IMT) measurements by ultrasonography according to LDL-C reduction. The relative risk reduction for stroke was 21% (odds ratio [OR] 0.79). Statin size effect was significantly associated with LDL-C reduction, as each 10% reduction in LDL-C was estimated to reduce the risk of all strokes by 15.6% and carotid IMT by 0.73% per year. Statins have become a mainstay in the management of atherosclerosis-related stroke events and, as already indicated, can significantly reduce stroke and stroke recurrence. However, there have been no recent randomized controlled trials comparing surgical and medical management with optimized medical therapy including statins to determine how their inclusion would modify surgical management algorithms.
Evaluation of plaque beyond the degree of luminal stenosis
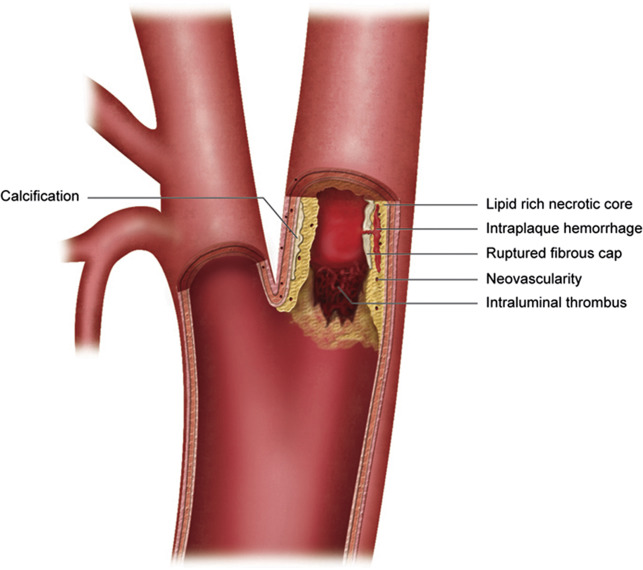
Coronary angiographic studies have indicated that moderate to low-grade arterial stenoses may lead to myocardial infarction, and frequently the most stenotic artery will not be upstream from the myocardial infarction. Subsequent histopathologic studies indicated that plaque erosion and disruption were common features in symptomatic lesions. Similar findings have been made with carotid plaques and associated cerebrovascular events. Lovett and colleagues found a significant association between carotid plaque surface irregularity on angiography and associated plaque rupture and histologic vulnerable plaque characteristics including intraplaque hemorrhage (IPH), lipid-rich necrotic core, and plaque instability. Histologic evaluation indicated that plaque erosion and rupture were frequently seen in these culprit lesions, indicating that stenosis was not the sole predictor of stroke risk. The degree of stenosis is a poor predictor of plaque volume and extent.
Inflammation is thought to represent an important destabilizing factor for atherosclerosis and is considered a major component of high-risk vulnerable plaque. Spagnoli and colleagues evaluated 269 carotid endarterectomy specimens, and found a significantly higher rate of inflammatory infiltrate and acute thrombus formation with associated fibrous cap rupture in patients with acute infarct when compared with patients with TIA or no symptoms. Seventy-four percent of infarct patients had a thrombotically active plaque (fresh clot composed of platelets and fibrin on the plaque surface) compared with 35% with TIA ( P <.001) and 14% of asymptomatic ( P <.001) patients. There was also a significant difference in the presence of cap rupture between stroke and TIA patients (67% vs 23%, P <.001), stroke and asymptomatic patients (67% vs 13%, P <.001) and TIA and asymptomatic patients (23% vs 13%, P = .004). The study also found that ruptured plaques in stroke patients had inflammation that was twice as dense as that seen in TIA ( P = .001) and asymptomatic ( P = .001) patients. Redgrave and colleagues evaluated 526 consecutive endarterectomy specimens, and found that dense plaque inflammation (with macrophage infiltration) was the feature most strongly associated with fibrous cap rupture (OR 3.9, P <.001) and time since stroke ( P = .001). There were significant negative associations between time since stroke and multiple plaque histologic features, including plaque macrophages ( P = .007), overall plaque inflammation ( P = .003), cap rupture ( P = .02), and overall plaque instability ( P = .001). There has been recent investigation of plaque inflammation and its correlation with plaque characteristics using PET/MR imaging. In the evaluation of 31 patients there was progressively significantly increased 18 F-fluorodeoxyglucose activity on PET imaging between thick, thin, and ruptured fibrous caps. Plaques with lipid-rich necrotic cores or IPH also had significantly higher metabolic activity than those predominantly composed of collagen or calcification.
Limitations of luminal imaging in evaluation of plaque volume
Atherosclerotic lesions frequently remodel outwardly in their early development, which allows for maintenance of luminal size even for prominent lesions. Glagov and colleagues showed that luminal encroachment occurs in coronary arteries once plaque occupies 40% of the area encapsulated by the internal elastic lamina. For this reason, luminal imaging is limited in its ability to detect early lesions and frequently underestimates plaque burden in more advanced disease. Underestimation of plaque burden by angiography has been confirmed by correlation with endarterectomy specimens. Furthermore, luminal imaging evaluates disease at the point of maximum stenosis in comparison with adjacent “normal” segments. However, this does not take into account the diffuse nature of atherosclerotic disease, which further contributes to the underestimation of disease burden.
Low-grade carotid lesions are often overlooked as a source of cerebral infarcts because of coexistent disease elsewhere, making it difficult to determine which lesion is the culprit and because plaque size is underestimated as a result of vascular remodeling. Identifying a plaque as having been the source of a stroke may change its risk profile for future events. Inzitari and colleagues showed that stroke risk in the territory of an asymptomatic carotid artery is substantially less than stroke risk in the territory of a symptomatic artery with a similar degree of stenosis. Dennis and colleagues showed that patients who experienced a TIA had a 13-fold excess stroke risk during the first year and a 7-fold excess risk over the first 7 years compared with those without TIAs. TIA might therefore be considered a warning for an impending cerebrovascular event and should warrant investigation of the culprit lesion.
There is a growing body of literature indicating that plaque features and lesion volume play an important role in the assessment of stroke risk, especially when there is little to no narrowing. Fig. 1 illustrates carotid plaque characteristics to be further discussed herein. Freilinger and colleagues prospectively evaluated 32 consecutive stroke patients with less than 50% ipsilateral carotid stenosis with vessel wall MR imaging. American Heart Association (AHA) type VI plaques were found in 37.5% of plaques ipsilateral to the symptomatic side, with none on the contralateral side ( P = .001). The most frequent vulnerable plaque features visualized included IPH (75%), fibrous cap rupture (50%), and intraluminal thrombus (33%). In reviewing 217 symptomatic patients with bilateral low-grade carotid stenosis, Cheung and colleagues found that the symptomatic side showed a significantly higher prevalence of IPH on T1-weighted vessel wall MR imaging than the contralateral side (13% vs 7%, P <.05). Altaf and colleagues evaluated patients with symptomatic mild carotid stenosis (30%–49%) and found that recurrence rates of ipsilateral stroke differed between patients with IPH represented by T1 hyperintensity (25%) compared with those without (10%). Yoshida and colleagues evaluated 25 patients with symptomatic low-grade (<50%) carotid stenosis and atherosclerotic plaque with high T1 signal and expansive remodeling on MR imaging. Eleven of the 25 patients had 30 recurrent infarcts on diffusion-weighted imaging (46% per patient-year) refractory to medical therapy, and were treated with carotid endarterectomy. Seven of the 11 patients in the recurrence group had no further stroke events in the postoperative follow-up period of 19.1 ± 14.6 months. Weinstein found that IPH and plaque ulceration on ultrasonography were strongly associated with symptoms despite many lesions showing less than 50% luminal stenosis. In the review of vessel wall MR imaging of 47 patients, Qiao and colleagues found that symptoms were significantly associated with IPH (OR 10.18, P = .03). There was a progressively increasing significant association between symptoms and extent of neovascularity as indicated by grades of adventitial enhancement (0 = absent, 1 = <50%, 2 = ≥50%) ( P = .02). The degree of stenosis did not correlate with ischemic events. These studies indicate how in the setting of low-grade or no stenosis, vulnerable plaque characteristics can determine the likelihood of symptoms and future events. There is continued investigation with multicenter trials to determine the role of vessel wall MR imaging in plaque characterization in the setting of cryptogenic stroke.
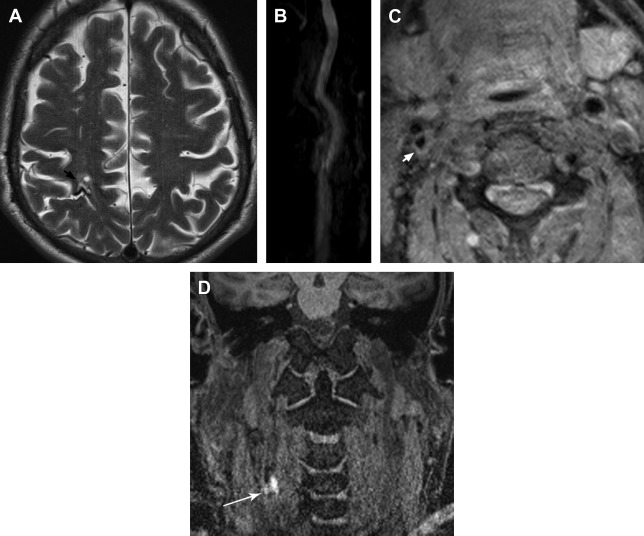
Plaque surface irregularity or ulceration has been found to be an important plaque vulnerability factor, significantly increasing the risk of stroke ( Fig. 2 ). In the analysis of 3007 patient angiograms from the European Carotid Surgery Trial, plaque surface irregularity was an independent predictor of ipsilateral ischemic stroke on medical treatment at all degrees of stenosis (HR 1.80, 95% CI 1.14–2.83; P = .01). In the evaluation of 659 patients who were found to have severe stenosis on angiography in NASCET, the risk of ipsilateral stroke at 24 months for medically treated patients with ulcerated plaques increased incrementally from 26.3% to 73.2% as the degree of stenosis increased from 75% to 95%. For patients with no ulcer, the risk of stroke remained constant at 21.3% for all degrees of stenosis. The net result yielded relative risks of stroke (ulcer vs no ulcer) ranging from 1.24 (95% CI 0.61–2.52) to 3.43 (95% CI 1.49–7.88). Catheter angiography is not sensitive or specific for the detection of plaque ulcerations. The sensitivity and specificity of detecting ulcerated plaques were 45.9% and 74.1%, respectively. The positive predictive value of identifying an ulcer was 71.8%. MR imaging/contrast-enhanced MR angiography is a sensitive technique for the detection of ulceration.
Plaque progression through repeated silent ruptures
Coronary plaques that may rupture will frequently be associated with only mild to moderate stenosis and have vulnerable plaque characteristics, similar to what can be seen with vulnerable carotid atheroma. The coronary plaques that rupture have a lipid-rich necrotic core and a thin fibrous cap rich in macrophage and T-cell infiltration with focal disruption. Reports of the mean necrotic core size seen with plaque rupture related to sudden coronary death range from 34% to 50%. These plaques are highly vascularized with extensive ingrowth of the vasa vasorum. Carotid atheromas follow a similar pattern of disruption, with fibrous cap foam cell infiltration, thinning, and neovascularity also influencing the likelihood of rupture.
Morphologic studies of coronary arteries suggest that plaque progression beyond 50% cross-sectional luminal narrowing occurs secondary to repeated ruptures, which may be clinically silent. The sites of healed plaque ruptures can be recognized by demonstrating a necrotic core with a discontinuous fibrous cap, which is rich in type I collagen, and an overlying neointima formed by smooth muscle cells in a matrix rich in proteoglycan and type III collagen.
Few angiographic studies have demonstrated plaque progression, and short-term studies have suggested that thrombosis is the likely cause. Mann and Davies showed that the frequency of healed plaque rupture increases along with lumen narrowing. Burke and colleagues found healed plaque ruptures in 61% of hearts from victims of sudden coronary death. Multiple healed plaque ruptures with layering were common in segments with acute and healed ruptures, and the percentage of cross-sectional luminal narrowing was dependent on the number of healed repair sites. The underlying percentage of luminal narrowing for acute ruptures exceeded that for healed ruptures (79% ± 15% vs 66% ± 14%; P<.0001). Therefore, the progression of atherosclerotic disease to severe stenosis is the result of repeated ruptures. At least 40% to 50% of coronary rupture sites show less than 50% diameter stenosis, and the same may be true in carotid disease. Spagnoli and colleagues reported a higher incidence of carotid thrombosis in patients with recent stroke in comparison with asymptomatic individuals.
High-resolution vessel wall MR imaging for carotid plaque component assessment
There has been increasing acceptance of noninvasive imaging for the evaluation of vulnerable plaque characteristics and their contribution to patient symptoms. Although ultrasonography has been proved to be a valuable technique for evaluation of plaque components, it is limited in its ability to differentiate IPH and lipid-rich necrotic core. Ultrasonography is sensitive for detecting calcifications but does a poor job imaging calcified plaques, as the acoustic shadowing limits soft-tissue visualization deep to the calcifications. Computed tomography (CT) imaging techniques can be used for plaque characterization, and provide a sensitive technique for the detection of calcifications. Some investigators have attempted to use lesion component attenuation for analysis ; however, attenuation characteristics depend on the energy level used and the administration of contrast, both of which can dramatically alter attenuation of lesion components. CT can also be of limited value in depicting some plaque components, including IPH. MR imaging has shown the ability to optimally differentiate plaque characteristics that confer plaque vulnerability, owing to its improved contrast resolution in comparison with CT and ultrasonography. Although CT is considered the best imaging technique for calcium detection in plaques, Clarke and colleagues reported MR imaging sensitivity of 97.6% for calcification detection compared with micro-CT and histology as the reference standard. MR imaging evaluation of calcification with histologic comparison showed MR imaging sensitivities ranging from 76% to 84% and specificity from 86% to 94%, with substantial agreement between the 2 techniques (κ = 0.65–0.75). Saam and colleagues determined the presence of calcification if there was matching plaque hypointensity on T1-weighted, T2-weighted, proton density–weighted, and time-of-flight (TOF) MR angiography sequences, with sensitivity of 76% and specificity of 86% when compared with histology. The sensitivity and specificity increased to 84% and 91% when only regions measuring greater than 2 mm 2 were considered. Puppini and colleagues used these same 4 MR sequences to evaluate for calcifications, and found strong agreement with histologic evaluation. Cappendijk and colleagues chose the best combination of contrast weightings from 5 different weightings to assess plaque calcifications among other plaque components, and found that on MR imaging 100% of plaque calcifications could readily be identified and differentiated from other plaque components relative to histologic evaluation.
There have been multiple reports of fibrous cap imaging on MR with histologic specimen correlation. Hatsukami and colleagues evaluated the fibrous cap on a 3-dimensional multiple overlapping thin-slab MR angiographic technique, and found good agreement on fibrous cap status (89% agreement, weighted κ = 0.87) with histologic findings. The fibrous cap was determined to be thick or thin based on the presence of a dark band between the bright lumen and gray wall. The cap was considered disrupted if the dark band was not visualized and there was bright gray signal adjacent to the lumen with or without an irregular luminal surface. Cai and colleagues used TOF MR angiography to determine fibrous cap status, differentiating between thick (>0.25 mm) or disrupted with similar determination of disrupted cap as described in the aforementioned study. There was good accuracy of MR imaging in identifying AHA type VI lesion characteristics including fibrous cap disruption, with sensitivity and specificity of 82% and 91%, respectively. Trivedi and colleagues defined fibrous cap as the juxtaluminal hyperintense component on short-tau inversion recovery sequences. These investigators compared MR imaging and histology-derived fibrous cap lipid-rich necrotic core thickness measurement ratios, and found strong agreement between the two, with a mean difference between the ratios of 0.02 ± 0.004. Mitsumori and colleagues used a multicontrast MR protocol to evaluate fibrous cap status, with an unstable cap represented by irregularity of discontinuity of the juxtaluminal hypointense band on TOF MR angiography, absence of intimal tissue between the lumen and deeper plaque structures, or focal contour abnormalities along the lumen surface. There was good agreement between imaging and histology for the evaluation of the fibrous cap. Detection of unstable fibrous cap on the multicontrast protocol had sensitivity of 81% and specificity of 90%. Wasserman and colleagues demonstrated improved detection of fibrous cap and outer wall boundary on MR imaging after contrast administration. Areas of increased enhancement within the cap may indicate areas of inflammatory infiltrate that may suggest impending rupture or neovascularity associated with plaque instability.
Clinical implications of plaque features
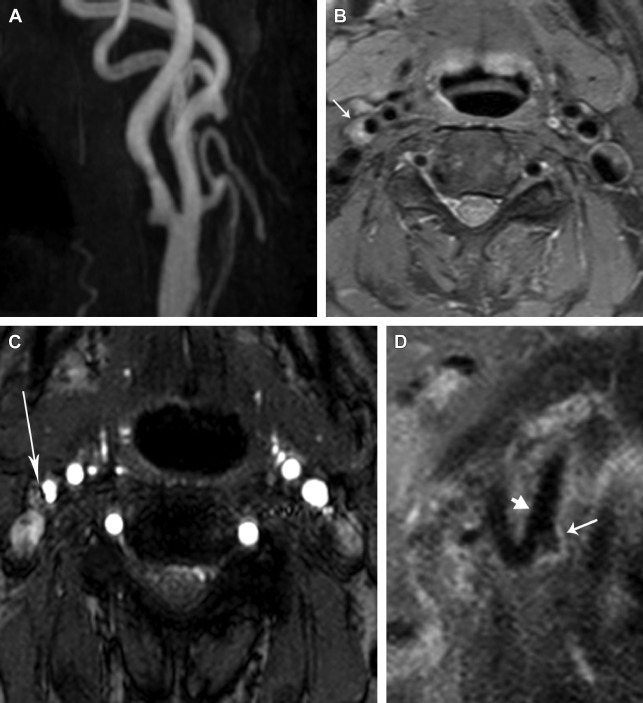
IPH has emerged as one of the most important atherosclerotic plaque features, contributing to plaque progression and leading to cerebrovascular ischemic events ( Fig. 3 ). In the setting of moderate asymptomatic carotid stenosis, Singh and colleagues reported a significant association between IPH and ipsilateral future cerebrovascular ischemic events (HR 3.59, 95% CI 2.48–4.71; P <.001). The presence of carotid IPH is associated with progression of plaque volume, increased lipid-rich necrotic core volume, and development of new intraplaque hemorrhages. During and after the development of IPH, the plaque growth rate is 18.3 ± 6.5 mm 3 /year, significantly higher than the plaque growth rate before IPH development (−20.5 ± 13.1 mm 3 , P = .018) and showed significant progression over baseline ( P = .008 compared with a slope of 0). Underhill and colleagues longitudinally imaged 67 asymptomatic patients with 16% to 49% carotid stenosis, and found that those with IPH showed significant progressive luminal narrowing when compared with those without IPH ( P = .005), and a progressive increase in plaque volume ( P <.001).