CHAPTER 15 Lower extremity veins
Duplex sonography
Color-coded sonography combined with Doppler waveform analysis (“duplex ultrasound”) is the principal imaging tool for evaluation of acute and chronic diseases of the lower extremity veins.1,2
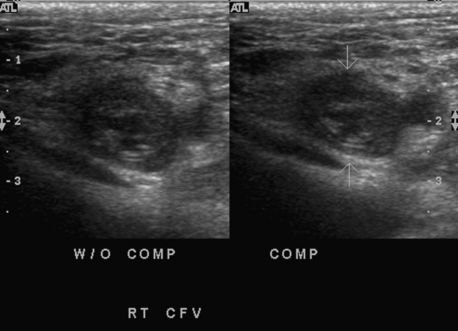
For detection of deep venous thrombosis, a high-frequency linear transducer (5 to 10 MHz) with color Doppler capability is used.3 The patient lies on a table; the leg is externally rotated and the knee slightly flexed. Progressing caudally from the groin, the veins are interrogated for complete color flow saturation of the lumen, which excludes thrombus. The more traditional but time-consuming incremental vein compression test is usually required only to confirm color Doppler flow pattern (Fig. 15-1). The popliteal vein is examined with the patient prone or in the lateral decubitus position. The study then is continued down the calf to assess the three paired tibial veins, preferably with the leg hanging over the examination table. Doppler waveform analysis is used to evaluate flow dynamics which may predict disease within vessels inaccessible to sonography (e.g., deep pelvic veins).
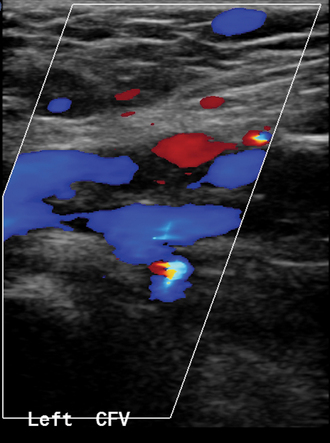
Similar equipment is used for evaluation of patients with suspected venous insufficiency or varicose veins. However, this study is performed with the patient standing and bearing weight on the opposite leg. The leg is slightly flexed at the knee. The great saphenous vein is interrogated from the groin to the level of the lowest varicosity.1 The vein diameters are measured, and the location of tributaries feeding any varicosities is noted. Following manual calf compression and release, venous reflux is sought from the confluence of the superficial inguinal veins through peripheral perforating veins with color imaging and Doppler waveform analysis (Fig. 15-2). The sine qua non of venous reflux is flow reversal lasting for a predetermined interval. Finally, the short saphenous vein and associated tributaries are examined from a posterior approach.
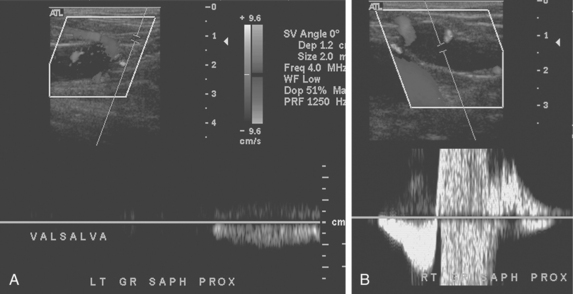
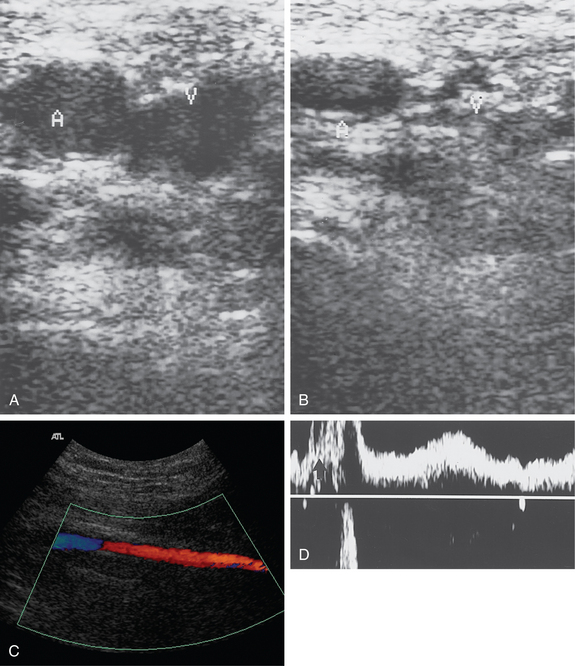
Figure 15-2 Venous reflux studies by duplex sonography. A, With a calf squeeze there is forward flow but no retrograde flow in the left great saphenous vein. B, In another patient, with a calf squeeze a burst of forward flow is followed by reversed flow greater than 0.5 second in duration in the right great saphenous vein, indicating venous reflux.
Anatomy
Normal anatomy and physiology
The leg is drained by superficial and deep venous systems. The deep veins, which are dominant, run alongside their corresponding arteries. The superficial veins are located in the superficial fascia. A network of perforating veins connects these two venous beds. The inconsistent and confusing nomenclature used over the years for lower extremity veins has recently been codified into a new set of standardized terms.4
In the foot, the deep plantar arch is formed by the plantar metatarsal veins.5 The arch forms the medial and lateral plantar veins, which give off branches to the saphenous system and contribute to formation of the tibial veins. The paired posterior tibial, anterior tibial, and peroneal veins constitute the deep system of the calf (Fig. 15-3). These tibial veins merge below the knee to establish the popliteal vein, which is usually superficial to the popliteal artery. Multiple muscular soleal and gastrocnemius veins also drain into the tibial and popliteal veins. The popliteal vein becomes the femoral vein (FV, formerly the superficial femoral vein) at the adductor canal (Fig. 15-4). It runs posterolateral to the artery above the knee and then posteromedial to the artery near the inguinal ligament. The deep femoral vein passes alongside the deep femoral artery and joins the FV at a variable distance below the inguinal ligament.
The superficial venous system includes the great saphenous vein (GSV), the small (formerly short or lesser) saphenous vein (SSV), and their numerous tributaries.4,5 The GSV begins anterior to the medial malleolus. The vessel ascends along the medial aspect of the calf and thigh superficial to the muscular fascia (Fig. 15-5). It then passes through the saphenous opening of the deep fascia and enters the medial side of the femoral vein (also receiving several pelvic venous tributaries) at the confluence of the superficial inguinal veins (formerly the saphenofemoral junction) (see Fig. 15-4). The SSV originates posterior to the lateral malleolus. It runs behind the heads of the gastrocnemius muscle bundles and, in most cases, joins the popliteal vein around the knee at the saphenopopliteal junction. In about one third of the population, the SSV communicates with the GSV directly via the intersaphenous (or Giacomini) vein or through another tributary.1
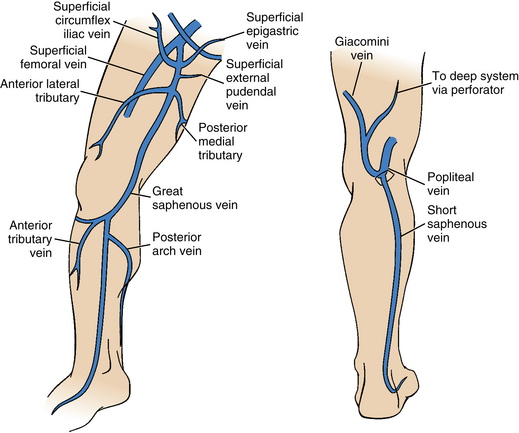
Figure 15-5 Superficial venous system of the leg.
(Adapted from Min RJ, Khilnani NM, Golia P. Duplex ultrasound evaluation of lower extremity venous insufficiency. J Vasc Interv Radiol 2003;14:1233.)
At the inguinal ligament, the common femoral vein becomes the external iliac vein, which is medial to the iliac artery (Fig. 15-6). Its tributaries include the pubic, deep circumflex iliac, and inferior epigastric veins. The external iliac vein courses over the pelvic brim to the level of the sacroiliac joint, where it joins the internal iliac vein to form the common iliac vein. The right common iliac vein is first posterior and then lateral to the iliac artery. The left common iliac vein has a more horizontal course and lies between the right common iliac artery and the spine. Asymptomatic mild extrinsic compression occurs at this site in up to 60% of the general population6 (see Fig. 15-6). Ascending lumbar veins arise from the upper surface of the common iliac veins. The iliac veins merge at the L4-L5 level to form the inferior vena cava (IVC).
When a person is supine, a pressure gradient (12 to 18 mm Hg on the venous side of capillaries; 4 to 7 mm Hg in right atrium) drives blood toward the heart.7 In addition, respiratory inspiration accelerates blood flow centrally. In an upright position, however, lower extremity venous drainage against substantial hydrodynamic resistance depends on the following: (1) an intact muscular pump, (2) competent vein valves, and (3) unobstructed outflow. Although the walls of the superficial and muscular leg veins contain smooth muscle and are capable of constriction, blood return to the heart depends largely on extrinsic compression by leg muscles, particularly the “calf pump.” At rest, blood flows from the superficial system into the deep veins. Competent valves prevent retrograde flow. With muscular contraction, blood is propelled centrally by forceful emptying of the deep veins.
Variant anatomy
Duplication of segments of the lower extremity veins is common. Accessory saphenous veins (which run in the extrafascial compartment) are described in up to two thirds of the general population.8 Close to one third of people have duplicated or multiple femoral (or less often, popliteal) veins.9
Klippel-Trénaunay syndrome (KTS) is a rare congenital disorder that features cutaneous capillary malformations, distorted lower extremity veins (multiple varicosities and often an ectopic marginal vein), and bone and soft tissue enlargement.10–12 A similar but distinct condition (Parkes-Weber syndrome) includes these elements along with high-flow arteriovenous malformations. Although most cases of KTS are sporadic and not familial, there is some evidence that the disease stems from genetic mutations in the angiogenic protein VG5Q.13
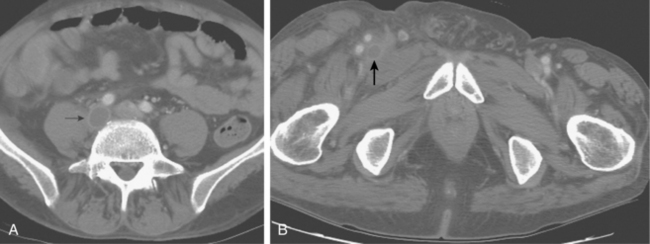
A cutaneous “port wine” lesion is characteristic. Symptoms include but are not limited to local pain, swelling, and bleeding. Sonography and computed tomography (CT) or magnetic resonance (MR) venography are useful in mapping the bizarre, disordered venous channels14 (Fig. 15-7). When present, the marginal vein runs along the lateral aspect of the thigh and drains into the internal iliac or femoral vein. Mild cases of KTS are managed with compressive stocking therapy alone. Sclerotherapy and embolotherapy are used to treat the abnormal venous channels and arteriovenous malformations associated with this syndrome.10 Surgery is reserved for those patients with functional disabilities or severe bleeding or cutaneous ulcerations.
Persistent sciatic vein is a very rare anomaly in which a remnant of that embryologic vessel remains the primary venous drainage between the adductor canal and the internal iliac vein.15,16 The vein is markedly dilated and tortuous. The disorder may be an isolated condition but often is associated with KTS.
Major disorders
Acute deep venous thrombosis (online case 85)
Etiology and natural history
Acute lower extremity and pelvic deep venous thrombosis (DVT) is part of the spectrum of venous thromboembolic disease (VTE, see also Chapter 1). This disorder also includes pulmonary embolism (PE) and chronic venous insufficiency (CVI, sometimes called post-thrombotic or postphlebitic syndrome).17,18 Acute DVT is a common clinical problem, with an overall lifetime risk of about 2% to 5% of the general population.19
In humans, thrombi are constantly forming in the valve sinuses and venous confluences of the muscular calf veins. The endogenous fibrinolytic system usually dissolves these small clots. Thrombus remains confined to the calf veins in about 75% of untreated patients.20 The risk of symptomatic PE in this situation is low.21 In the remaining cases, thrombus extends to the “proximal” (femoropopliteal) veins. Most of these individuals have more than one predisposing factor to clot formation, such as sluggish blood flow, vessel injury, or a thrombophilic state (i.e., Virchow triad) 7,22 (Box 15-1). Relative hypercoagulability is the leading culprit in most instances.
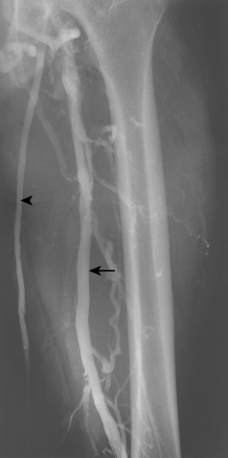
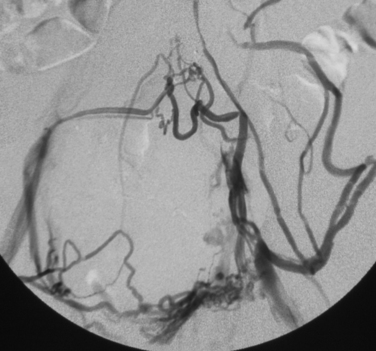
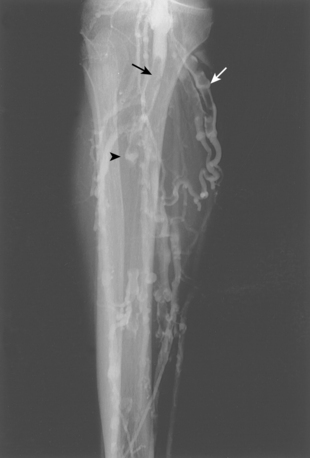
Iliac vein thrombosis can occur with propagation of femoropopliteal vein clot. Isolated iliofemoral venous thrombosis (encompassing the common femoral and iliac veins) accounts for no more than 20% of cases of lower extremity DVT and usually can be attributed to local factors23 (Fig. 15-9 and Box 15-2). Left iliac vein compression (May-Thurner syndrome) frequently is responsible for left-sided iliofemoral thrombus formation (see later discussion). Iliofemoral DVT is also remarkable for the rarity of complete recanalization, relative resistance to conventional anticoagulative measures, and more profound (and perhaps frequent) symptoms of post-thrombotic syndrome (PTS).24
In a small subset of patients, extensive peripheral and iliofemoral thrombosis occurs, leading to an edematous, painful white leg (phlegmasia alba dolens). If obstruction of collateral channels ensues, venous drainage is almost completely blocked and the patient will suffer from a markedly swollen, painful cyanotic extremity, sometimes with diminished arterial pulses (phlegmasia cerulea dolens).25 This grave condition (which requires immediate intervention) may lead to venous gangrene, massive PE, or death.
Once acute DVT occurs, the fate of the thrombus is variable7,26 (Box 15-3). The likelihood of a particular outcome depends on many factors, but some important generalizations can be made:
Diagnosis
Although individual clinical signs often are misleading, “predictive rules” such as those devised by Wells and colleagues have proven valuable in assessing risk for the disease (Box 15-4). Because the consequences of a missed diagnosis and no treatment can be life-threatening, screening of high-risk asymptomatic patients and accurate diagnosis of symptomatic patients is critical and must depend on risk factor analysis, objective diagnostic tests, and imaging studies, alone or in combination.
Box 15-4 Clinical Predictors of Acute Deep Venous Thrombosis
Data from Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003;349:1227.
D-dimer is a byproduct of endogenous fibrin degradation, and plasma levels are elevated in patients with venous thrombosis or PE. The D-dimer radioimmunoassay has been useful in excluding acute DVT, particularly when applied along with predictive rules.19,34,35 For example, a negative result from a high-sensitivity D-dimer test (e.g., serum level <500 ng/mL) and a low Wells score puts the likelihood of acute DVT diagnosis within 3 months at about 0.5%.36
Imaging
Sonography
Because duplex sonography is extremely accurate, easy to perform, relatively inexpensive, and completely safe, it is the principal imaging study in both symptomatic and asymptomatic groups.37–40 Several factors are evaluated (Fig. 15-10 and Table 15-1). Sonography also detects other causes for leg symptoms, such as a Baker cyst, lymphadenopathy, hematoma, or popliteal artery aneurysm.
Table 15-1 Color Doppler Sonography of Acute Deep Vein Thrombosis
| Factor | Normal Finding |
|---|---|
| Color flow | Complete saturation |
| Compressibility with transducer | Complete coaptation of vein walls (at accessible sites) |
| Phasicity | Decreased flow with inspiration, increased with expiration (reflects patency of central veins) |
| Provocative maneuvers | Flow increased with calf compression, decreased with Valsalva (reflects patency of central veins) |
In symptomatic patients, compression and color Doppler sonography are about as accurate as traditional contrast venography in the diagnosis of acute proximal DVT, with a sensitivity of 89% to 96% and specificity of 94% to 99%.37–40 Outcome analyses from two large cohorts of patients with suspected DVT estimated the negative predictive value of a normal compression sonogram at greater than 99.5%.41,42
In asymptomatic patients with risk factors for DVT, color Doppler sonography is not as accurate (sensitivity 47% to 62%).38,43 This discrepancy largely is related to the nature of occlusions in this population (i.e., often confined to calf veins, composed of smaller, nonocclusive, segmental thrombi).
Misdiagnosis is usually related to underlying chronic venous disease, isolated clot (iliac vein, calf veins, deep femoral vein, or one limb of a duplicated femoral or popliteal vein) and technical errors. Acute and chronic DVT can be confused at sonography. Certain features are used to differentiate between these entities (Table 15-2). Sometimes, however, it is impossible to distinguish among an acute clot occupying a previously normal vein, a chronic organized clot, and acute clot superimposed on chronic disease (Fig. 15-11). It is for this reason that some experts recommend a follow-up sonogram as a baseline study for all patients with acute DVT in the event that symptoms recur.
Table 15-2 Sonographic Distinction Between Acute and Chronic Deep Venous Thrombosis
| Feature | Acute DVT | Chronic DVT |
|---|---|---|
| Lumen | Dilated | Narrowed or normal caliber |
| Luminal flow | Absent or minimal | Residual flow, reflux |
| Vein wall | Thin | Thickened |
| Collaterals | Poorly developed | Well developed |
| Compression | Spongy | Firm resistance |
DVT, deep venous thrombosis.
The clinical significance of isolated calf vein DVT is controversial.28,44,45 Compression sonography and color Doppler flow imaging are fairly accurate in detecting calf vein clot.46,47 In some series, the sensitivity of color Doppler sonography is comparable in the calf veins and proximal veins. But in routine practice, technically inadequate studies of the calf are relatively common.40,48 Some practitioners opt for serial examinations of the proximal veins to identify propagation into the femoropopliteal system.
Computed tomography imaging
Even though CT venography of the lower extremities is relatively accurate, it is rarely employed as the initial step in routine cases. Some centers favor combined CT venography and CT pulmonary angiography in patients with suspected venous thromboembolic disease.49 CT venography is more accurate than sonography in the pelvic veins and IVC and may identify the mechanism for isolated iliofemoral DVT.50–53 Typical findings are luminal filling defects, vein wall enhancement, and filling of collateral veins (see Fig. 15-9). Incomplete opacification of vein segments and beam-hardening artifacts can mimic clot.
Magnetic resonance imaging
The accuracy of MR venography in the diagnosis of DVT in the leg is similar to contrast venography and duplex sonography.54–56 However, an MR examination is more costly and time consuming than a sonogram. Still, MR imaging (MRI) is superior to sonography for pelvic vein and IVC disease.
Ascending venography
Although catheter-based contrast venography was once considered the gold standard in the diagnosis of acute and chronic DVT, in fact it was not always reliable.57 One autopsy study revealed a sensitivity and specificity of 89% and 97%, respectively.58 Interobserver variability is at least 10% to 15%.59 The procedure is uncomfortable for the patient, and it has a small risk of significant complications. For these reasons, it rarely is used for diagnosis anymore.
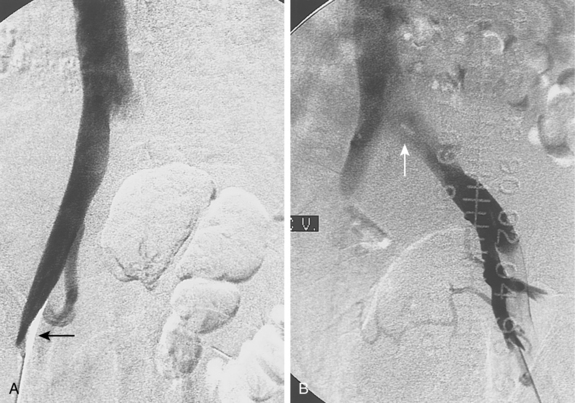
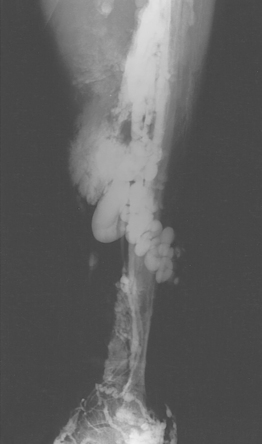
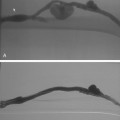
A fresh clot produces an intraluminal, wormlike filling defect (Fig. 15-12). Abrupt occlusion of a vein can reflect acute or chronic thrombosis (see Fig. 15-8). Nonfilling of deep veins, preferential filling of superficial veins, and opacification of collateral veins may occur with acute or chronic DVT. Thrombus may be confused with other causes for nonfilling of portions of the deep venous system.
Imaging strategy
Every physician must develop his or her own algorithm for assessing symptomatic and high-risk patients for acute DVT using a combination of risk-factor analysis, lab testing (D-dimer), color Doppler sonography, and other imaging studies. Periodic surveillance of at-risk populations has uncovered lower extremity DVT in up to 28% of patients, about 80% of whom were asymptomatic.60–62 Many clinicians order follow-up exams after a normal ultrasound in all cases or at least in those with moderate to high clinical probability of disease. However, only 1% to 2% of patients with initially negative studies subsequently are shown to have developed proximal DVT.63,64 D-dimer results may be used to withhold ultrasound in patients with low clinical probability.37 A follow-up study after completion of anticoagulant therapy may be advantageous in higher-risk patients should symptoms recur in order to distinguish chronic disease from recurrent acute DVT.
Treatment
Patients with acute DVT are treated to prevent or limit the major sequelae of venous thrombosis: PE, CVI, and recurrent DVT.28,65,66
Medical therapy
The cornerstone of treatment is anticoagulation. In addition, class II (30 mm Hg) elastic compression stockings are strongly recommended to lessen the risk for PTS.67 Anticoagulation does not directly promote fibrinolysis, but it allows endogenous lysis to occur and reduces the likelihood of PE by limiting clot propagation. For many decades, treatment for DVT consisted of unfractionated intravenous heparin (3 to 5 days) and oral warfarin (at least 3 to 6 months).28,68 In most centers, low molecular weight heparin (e.g., enoxaparin, tinzaparin) or a specific factor Xa inhibitor (e.g., fondaparinux) has replaced initial heparin therapy. Low molecular weight heparins have a more predictable response and a longer duration of action, and they can be given at home once or twice daily as a subcutaneous injection66,68 (see Chapters 2 and 3). Monitoring of drug effect is unnecessary except in certain situations (e.g., obesity, pregnancy, heart or liver failure). Therapeutic anticoagulation substantially reduces the likelihood of PE. Even with full compliance, however, recurrent DVT still occurs in more than 25% of cases, and at least one fourth will ultimately suffer from chronic PTS.31,65,69,70
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree