Key Points
- •
Ultrasound guidance for therapeutic thoracentesis can eliminate needle trauma as the immediate cause of postprocedural pneumothorax.
- •
Site-marking techniques achieve high safety and success rates as long as the patient is not repositioned before needle insertion.
- •
Ultrasound–guided biopsy of peripheral lung masses can achieve higher histologic yield than CT guidance because of the ability of ultrasound to differentiate solid from liquid areas.
Background
Ultrasonographic guidance of thoracic drainage and biopsy is an attractive alternative to computed tomography (CT) or fluoroscopic guidance given the avoidance of radiation exposure and need for specialized procedure suites. Providers performing thoracic interventions must possess the skills required to perform pleural and lung ultrasound, and in the case of anterior mediastinal biopsy, must be familiar with the anatomy of the anterior chest wall and mediastinum.
Equipment
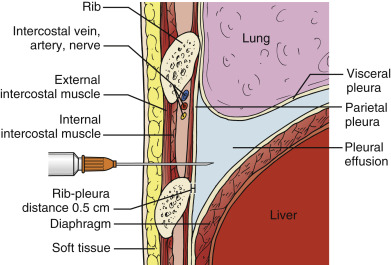
Phased-array or micro-convex array transducers with frequencies between 2 and 5 MHz (typically 3.5 MHz) are suitable for most pleural drainage procedures. High-frequency, linear transducers should be used to biopsy peripheral nodules or masses due to better near-field resolution and improved spatial localization of the needle tip. Combining ultrasound imaging with traditional bony landmark detection (i.e., finding the upper rib margin with a finder needle before insertion of a catheter for fluid drainage) minimizes the risk of injury to the intercostal neurovascular bundle ( ![]() ). The transducer orientation marker is pointed cephalad, and the corresponding screen indicator is placed on the upper left side of the image to maintain spatial relationships between the target lesion and surrounding structures.
). The transducer orientation marker is pointed cephalad, and the corresponding screen indicator is placed on the upper left side of the image to maintain spatial relationships between the target lesion and surrounding structures.
Patient Positioning
Free-flowing pleural effusions collect in the lateral and posterior costophrenic recesses and are most readily accessed from the posterior chest wall of a seated patient. The approach to positioning critically ill patients varies with the size of the effusion, body habitus, type of support devices, and physiologic compromise, such as hemodynamic instability or respiratory failure. Large effusions may be accessed with the patient in a supine position. Positioning the ipsilateral arm across the chest by securing the hand to the contralateral bedrail greatly improves lateral access and helps maintain patient position throughout the procedure. Patients may also be supported in a sitting position on the edge of the bed, or placed in a full lateral decubitus position. Patient positioning for biopsy of lung or pleural lesions depends on the location of the lesion. Both the patient and operator should be comfortable to maintain their position for the duration of the procedure, which is critical to procedural success and maintenance of sterility. A prone position is ideal for posterior lesions if the patient can tolerate this position. Overall, the most important principle of positioning for ultrasound-guided procedures is to individualize the approach by taking advantage of the flexibility of ultrasound imaging to maximize patient and operator comfort.
Thoracentesis
Ultrasound guidance of thoracentesis increases procedural success rate and decreases mechanical complications. The success rate depends on eliminating inadvertent attempts to drain fluid when no or minimal fluid is present. Therefore, proficiency in diagnostic sonography of pleural effusions is a prerequisite to performing ultrasound-guided thoracentesis. The most common complications of thoracentesis include pneumothorax, pain, shortness of breath, cough, and vasovagal reaction. Other complications that have been described are reexpansion pulmonary edema, inadvertent liver or splenic injury, hemothorax, infection, subcutaneous emphysema, air embolism, and chest wall or subcutaneous hematoma. Of all the complications, ultrasound guidance appears to lower rates of traumatic pneumothorax after thoracentesis from a range of 5–18% with a landmark-based approach to 1–5% with an ultrasound-guided approach. In our experience, the rate of pneumothorax is near zero when pneumothorax ex vacuo is excluded.
Ultrasound guidance for therapeutic thoracentesis appears to almost eliminate needle trauma as the immediate cause of postprocedural pneumothorax in spontaneously breathing patients. Pneumothorax in this setting has been reported to be associated with unexpandable lung and not laceration of the visceral pleura in almost all cases. Thoracentesis is an ideal procedure for novice providers to learn how to use ultrasound to establish a suitable needle access site and avoid organ puncture.
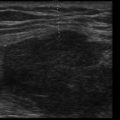
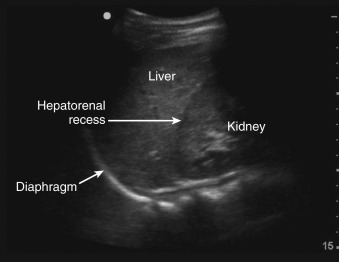
Selecting a suitable needle insertion site requires demonstration of pleural fluid immediately adjacent to the parietal pleura and sufficient distance from the diaphragm and lung throughout the respiratory cycle. The diaphragm, liver, and spleen should be identified unequivocally. The curvilinear line of the hepatorenal recess (Morison’s pouch), located between the liver and kidney, should not be confused with the diaphragm ( Figure 11.1 ).Misidentification of these lines can result in hepatic or splenic injury, especially when gain is not appropriately adjusted.
Once the access site is marked, site verification and needle insertion angle should be simulated as follows. The operator should place the transducer firmly against the skin with the transducer angled along the same trajectory as the anticipated needle insertion path, and then the operator should look at the screen to ensure the center of the resulting image is devoid of all organs including lung. Color flow Doppler may be applied along the anticipated needle trajectory to ensure avoidance of any collateral arteries along the upper rib surface. An important advantage of using ultrasound guidance is the ability to measure distances between tissues, namely, between the skin, parietal pleura, and visceral pleura ( Figure 11.2 ). Provided the patient does not change position or cough during the procedure, these distance measurements are reliable to determine an appropriate needle insertion depth to avoid touching the diaphragm or lung. Measurement of chest wall thickness is subject to compression artifact, and providers should release transducer pressure on the skin before freezing the image to take measurements. A minimum pleural effusion depth of 1.5 cm is recommended to safely perform a thoracentesis. After the operator advances the needle over the upper margin of the rib, pleural fluid return is expected approximately 5 mm beyond the rib ( Figure 11.3 ). The needle lumen can be occluded by blood clot or solid debris of a complex effusion. If occlusion is suspected the needle lumen can be cleared by injecting small amounts of local anesthetic or saline. In addition to sonographic localization of pleural fluid, a lateral distance of at least 6 cm from the spinous processes should be maintained to minimize the risk of injury to the intercostal neurovascular bundle.