Lung and Thorax
Calvin Huang
Andrew S. Liteplo
Vicki E. Noble
INTRODUCTION
Before the emergence of point-of-care ultrasound, the evaluation of thoracic complaints comprised history, physical exam findings, including lung auscultation and percussion, x-ray imaging, and chest computed tomography (CT). Apart from pre-procedural marking for thoracentesis, ultrasound applications were initially limited to evaluating solid organs or fluid-filled spaces, typically the abdomen and vascular structures. Even then, it was common for marking to occur in the radiology suite, and for thoracentesis to be performed at the bedside by another medical team. Thoracic ultrasound was perceived to be limited due to the relative inability of sound waves to pass through regions of air. The drastic change in tissue impedance when sound waves transition from soft tissue to air causes scatter, resulting in the loss of a traditional organ image. However, point-of-care sonographers have recognized that while the lung tissue itself is often visualized poorly, the presence or absence of specific artifacts caused by this large change in impedance gives valuable clinical information, often faster and more accurately than traditional imaging modalities. Research and acceptance of this very useful ultrasound application has since rapidly grown.
CLINICAL APPLICATIONS
Thoracic ultrasound is extremely useful in the emergency and critical care setting, not only because it can be performed in a timely fashion at the bedside and repeated infinitely without risk of radiation, but also because, in many instances, it has been shown to be more sensitive and specific than radiation-based imaging (1, 2, 3, 4, 5). There are four main categories of thoracic ultrasound diagnoses: (1) pleural-based diseases (pneumothorax), (2) interstitial-based diseases (pulmonary edema, fibrosis), (3) consolidative diseases (acute respiratory distress syndrome (ARDS) and pneumonia), and (4) effusions.
Pneumothorax is the primary diagnostic question for most point-of-care sonographers when evaluating the pleura. Evaluating this diagnosis is one of the most common questions critical care clinicians have, and ranks high in the life-threatening differential diagnosis in any patient with chest pain or dyspnea. Pneumothorax can be spontaneous, traumatic, or iatrogenic. Iatrogenic causes include direct injury during internal jugular or subclavian line placement, as well as barotrauma from positive pressure ventilation. Point-of-care thoracic ultrasound can quickly and effectively evaluate for pneumothorax.
Thoracic ultrasound can also examine for interstitial fluid. The constellation of pathology that can lead to this fluid accumulation is referred to as interstitial syndrome, and includes congestive heart failure (CHF), ARDS, fibrotic processes, and early pulmonary contusion or infarct. The presence of interstitial fluid on thoracic ultrasound, in combination with secondary ultrasound findings and an understanding of the clinical picture, can lead to the correct diagnosis.
Pleural effusion has multiple etiologies, including trauma, malignancy, CHF, and hepatorenal failure. The presence of pleural effusion can be evaluated in both the upright and supine patient. This imparts benefit when compared to radiography in that some patients are unable to
lie flat for traditional imaging due to dyspnea and some patients who are critically ill or intubated cannot sit up for a chest x-ray. Furthermore, bedside localization with ultrasound of fluid allows for simple transition into procedural guidance.
lie flat for traditional imaging due to dyspnea and some patients who are critically ill or intubated cannot sit up for a chest x-ray. Furthermore, bedside localization with ultrasound of fluid allows for simple transition into procedural guidance.
Finally, lung ultrasound can also be helpful in the detection of lung consolidation. Even further, when consolidation is detected on chest x-ray, secondary sonographic signs can help differentiate between the different causes of consolidation, which include atelectasis, pneumonia, and pulmonary embolus (PE).
IMAGE ACQUISITION
The technique for image acquisition depends upon the specific clinical indication for which thoracic ultrasound is being performed. The depth, type of transducer, and scanning protocol are determined by the clinical question.
Pneumothorax
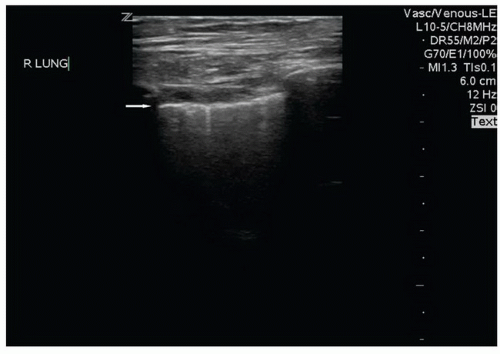
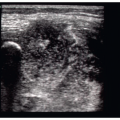
The presence or absence of pneumothorax is commonly evaluated with the patient in a supine position. The high-frequency linear probe is placed perpendicular to the ribs on the anterior chest. This region is targeted because air in the pleural space is likely to rise anteriorly in the supine patient. The ribs and their shadows will be seen in a short axis view, and they should be placed at the edges of the window (Fig. 5.1). The depth should be adjusted so that the pleural line is centered in view. M-mode imaging can be employed for additional confirmation and is described below. The M-mode axis should be set perpendicular to the pleural line, midway between two rib shadows (Fig. 5.2).
Multiple anterior zones, as well as the apical region, should be scanned to increase sensitivity. In previously normal lung, pleural adhesions are rare, and as such, the likelihood of a loculated pneumothorax is low. However, in patients with prior abnormal lung pathology, pneumothoraces can be loculated. The lateral and posterior lung fields should be scanned when concern for pneumothorax is high as previous scarring may prevent air from moving to the most anterior or superior location.
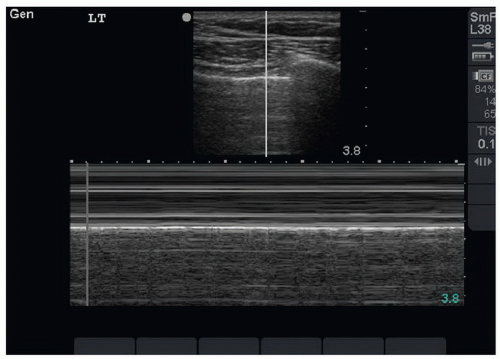 FIGURE 5.2. M-Mode Line should be Placed Midline between the Two Rib Shadows. Seashore sign is present, indicating a normal lung (no pneumothorax). |
Because the pleural line is only a few centimeters below the skin surface, the linear probe is generally employed. However, the lower frequency curvilinear or phased array probe may be utilized without any change in technique, and may be necessary in patients with a large amount of subcutaneous tissue. However, the curvilinear probe is generally pre-set to view deeper structures and as such, the focus depth will usually need to be decreased.
Interstitial Fluid or Lung Consolidation
The location of interstitial fluid or lung consolidation is dependent upon the pathology and as such, can be diffuse (severe CHF, interstitial pneumonia, pulmonary fibrosis) or focal (pneumonia, early CHF, atelectasis, pulmonary contusion/infarct). Scanning can be performed in the seated or supine patient; in patients with severe orthopnea, a supine position might not be feasible. There are multiple scanning protocols described, and they range from a rapid sweep of the probe anteriorly to evaluating 26 distinct zones of the thoracic cavity. Obviously, different clinical scenarios will call for different levels of sensitivity, and in general, the more zones that are evaluated, the more sensitive and specific the exam for identifying and qualifying interstitial fluid (6, 7, 8). The most common scanning protocol in the emergency department (ED) is to investigate eight zones—superior and inferior zones of the anterior and lateral chest on both sides (Fig. 5.3) (6).
A low-frequency probe is selected for this exam—either a phased array or a curvilinear probe. Because B-lines are defined as reverberation artifacts that extend radially from the pleural line to a depth of 18 cm, the field depth for interstitial fluid must be set to at least this extent when evaluating for interstitial fluid. Lung consolidation can vary in depth, and the depth should be adjusted dynamically to obtain the optimal view. The probe is placed perpendicular to the ribs so that they are viewed in short axis. The pleural line should be centered and in view between two rib shadows.
Pleural Effusion
Pleural effusion can be detected in the seated or supine patient. In the seated patient, gravity-dependent fluid is best
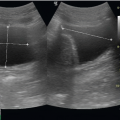
visualized from a posterior approach. Fluid will accumulate on the diaphragm. The linear or curvilinear probe is utilized and held in the sagittal plane with the probe marker pointing toward the patient’s head. When evaluating either the right or the left hemithorax in the seated patient, the probe should be placed at the posterior costal margin in the mid-scapular line and the diaphragm identified. If fluid is seen, there will be an anechoic stripe separating the visceral and parietal pleural (Fig. 5.4). The height of the effusion can be evaluated by sliding the probe in a cephalad direction until the normal pleural line reconnects.
visualized from a posterior approach. Fluid will accumulate on the diaphragm. The linear or curvilinear probe is utilized and held in the sagittal plane with the probe marker pointing toward the patient’s head. When evaluating either the right or the left hemithorax in the seated patient, the probe should be placed at the posterior costal margin in the mid-scapular line and the diaphragm identified. If fluid is seen, there will be an anechoic stripe separating the visceral and parietal pleural (Fig. 5.4). The height of the effusion can be evaluated by sliding the probe in a cephalad direction until the normal pleural line reconnects.
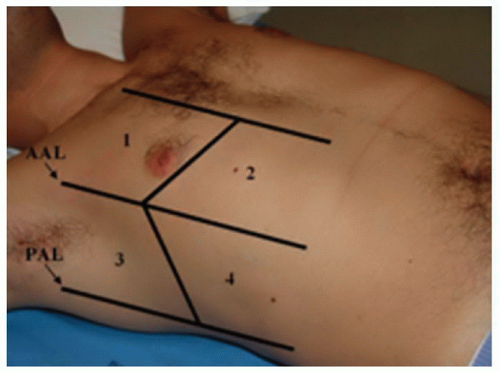 FIGURE 5.3. Picture of the Eight Zones (the Ones Shown Plus the Four Contralateral Zones) Commonly Used to Identify the Presence or Absence of Interstitial Fluid. |
In the supine patient, gravity-dependent fluid will accumulate posteriorly and inferiorly, and as such, the largest pocket of pleural fluid will be located superior to the diaphragm. The curvilinear probe should be utilized. In the right upper quadrant of the abdomen/right lower chest the probe should be positioned in the coronal plane, in the mid-axillary line near the level of the costal margin. The probe marker will point toward the patient’s head. The liver parenchyma should be visualized, and superior to this, the diaphragm. The depth should then be decreased so the liver/diaphragm interface is maximized in the window. On the left chest the interface between the spleen and the diaphragm is more superior and posterior when compared to the right. As such, the probe is best positioned at the posterior axillary line and adjacent to the costal margin.
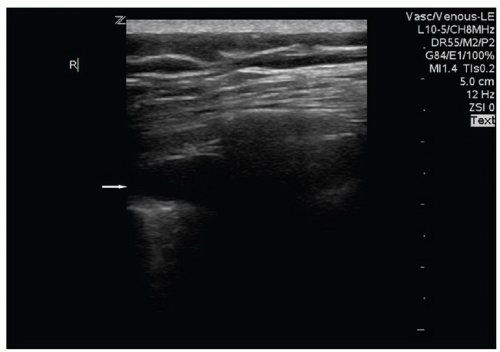 FIGURE 5.4. Pleural Effusion (Arrow) Present with the Parietal and Visceral Pleura Separated by the Hypoechoic Fluid. |
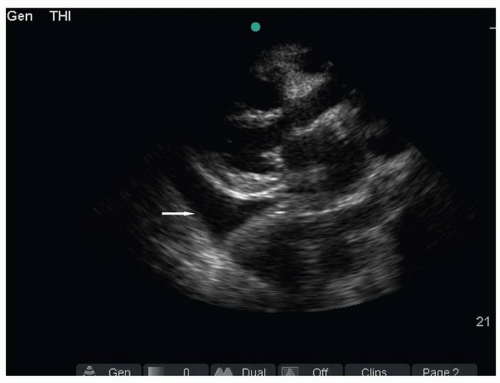 FIGURE 5.5. Image of Pleural Effusion (Arrow) in the Parasternal Long Axis View. The fluid tapers, is posterior to the descending thoracic aorta, and does not cross the midline. |
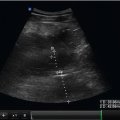
Of note, large pleural effusions can also be detected in the supine patient when obtaining parasternal cardiac views. While there are no studies comparing the sensitivity of the different acoustic windows, because fluid is gravity-dependent, a small pleural effusion is unlikely to be detected from the anterior parasternal view. It is more common that pleural fluid is detected as an incidental finding, prompting further thoracic evaluation. With the phased array probe, the parasternal long axis view is obtained by placing the transducer just lateral to the left sternal border at the fourth or fifth intercostal space. The probe axis is held in a line connecting the right shoulder and left hip. Once the cardiac window is obtained, a pleural effusion may be visualized posterior (far field) to the descending thoracic aorta (Fig. 5.5).
NORMAL ULTRASOUND ANATOMY
In the normal thorax, the interior chest wall and lung parenchyma are surrounded by a continuous membrane, which comprises the visceral pleural as it lines the lung, and the parietal pleura as it lines the interior of the chest cavity. These two layers are in constant contact and slide back and forth against each other as the lung and chest wall expand and contract. The pleural space contains several milliliters of fluid, which acts as a lubricant and facilitates sliding.
The basic ultrasound view of the pleural space is called the bat sign (9). It is obtained with the probe axis perpendicular to the rib shadows. The pleural line is visible as a hyperechoic line parallel to the transducer surface, deep to the anterior surfaces of the rib (Fig. 5.1). The pleural line comprises the body of the “bat,” and the anterior rib surfaces with underlying shadows generate the wings. Recognizing the bat sign is key when identifying the relevant anatomy.  PEDIATRIC CONSIDERATIONS: Because of reduced reflection of ultrasound through pediatric bones, the pleural line is readily visible below the ribs. The pleural sliding is often even more noticeable in these areas.
PEDIATRIC CONSIDERATIONS: Because of reduced reflection of ultrasound through pediatric bones, the pleural line is readily visible below the ribs. The pleural sliding is often even more noticeable in these areas.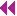
 PEDIATRIC CONSIDERATIONS: Because of reduced reflection of ultrasound through pediatric bones, the pleural line is readily visible below the ribs. The pleural sliding is often even more noticeable in these areas.
PEDIATRIC CONSIDERATIONS: Because of reduced reflection of ultrasound through pediatric bones, the pleural line is readily visible below the ribs. The pleural sliding is often even more noticeable in these areas.
Lung sliding can be visualized sonographically and indicates that there is no pneumothorax. As the visceral and pleural parietal layers slide past one another, the pleural line will be seen moving back and forth horizontally. Reverberation artifacts, known as B-lines, may also be seen emanating from the pleural line. While these may indicate pathology in the interstitial spaces, the presence of comet tails or B-lines rules out a pneumothorax because air between the two pleura prevents the reverberation artifact between the two pleura ( VIDEO 5.1).
VIDEO 5.1).
 VIDEO 5.1).
VIDEO 5.1).Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree