Lung Cancer
Patrick J. Peller
Val J. Lowe
Lung cancer is the most common cancer in the United States with an estimated 175,000 new cases and 163,000 deaths annually. Lung cancer death rates have begun to plateau recently in the United States, particularly in men (1). There are more than 600,000 deaths worldwide from lung cancer, and this rate is expected to rise for the next two decades and quite likely even longer (2). Lung cancer deaths in the United States exceed all those in breast, prostate, and colorectal cancer patients combined. In the past 10 years, there has been decreasing mortality in breast, prostate, and colorectal cancer patients due, in part, to increased screening and improved diagnosis and treatment, but no similar significant trend has been seen with lung cancer (3).
Smoking is closely linked to lung cancer, and historically the incidence of lung cancer parallels the availability of mechanically produced cigarettes (4). In the United States today, there are 25.5 million male smokers and 21.5 million female smokers, representing just less than 24% of the population. The incidence of smoking remains higher in Native Americans and black Americans than in the general population. The overall incidence of smoking has declined slowly over the past decade, but in certain populations, especially teenagers and those under the poverty level, there is concern for high and rising smoking rates (5). Prevention of lung cancer and smoking are inexorably tied and continues to be a major health battle.
Significant advances in the management of lung cancer have been seen over the past decade. Traditional computed tomography (CT) has substantially changed with low-dose CT screening and especially with multidetector CT. Surgery remains the mainstay for lung cancer therapy, but multimodality treatment is increasingly common as many cancers are first diagnosed after they are too large or too disseminated for surgical management. New chemotherapy agents and regimens are available, especially targeted treatments such as antiangiogenesis and epidermal growth factor receptor agents. Positron emission tomography (PET) scanning continues to evolve in use as there is increasingly common reimbursement for PET and PET/CT at multiple stages within the course of the disease. Significant technical developments in PET, such as new lutetium oxyorthosilicate (LSO), gadolinium silicate (GSO), and lutetium yttrium orthosilicate (LYSO) detector systems, improved electronics, three-dimensional imaging, time-of-flight PET, respiratory gating, and integration with CT have changed the face of PET imaging in many ways. Widespread availability of commercially prepared fluorodeoxyglucose (FDG) and PET scanners has brought access to PET to most patients with lung cancer in the United States, although patterns of use differ among practitioners. PET has multiple roles in patients with known or suspected lung cancers. Although PET with FDG is the focus of this chapter, it should be realized that PET with carbon-11-L-methionine, used commonly in Japan and in other centers with cyclotrons, also performs quite well in staging and assessing lung cancer.
Evaluation of Pulmonary Nodules
Solitary pulmonary nodules (SPN) are a significant clinical problem and are predominantly detected incidentally on chest x-ray or CT. Traditionally, SPNs were nodules 1 to 3 cm in size. More than 75% of the patients are asymptomatic at the time of detection (6). The major clinical question is what is the etiology of this nodule, benign or malignant? This leads to asking if the SPN should be followed or resected. The evaluation becomes risk assessment, which becomes the “cost” of resecting a benign nodule versus the “cost” of missing a malignancy. Multidetector CT has started to make the “solitary” part of SPN a disappearing entity and adds complexity, as the SPN on chest x-ray becomes one of many nodules being detected, although most of the nodules are small (<1 cm). Thus, work-up of nodules less than 1 cm in size is more common than it once was.
Attacking the question of a pulmonary nodule has led to a wide variety of management strategies. All strategies clearly start with establishing the presence or absence of the nodule on a prior chest x-ray or CT; size stability over a 2-year time period is accepted as evidence of benignity. Unfortunately, it is often the case that no prior imaging studies are available, and the stability of lesion size cannot be confirmed. Otherwise, a three-pronged individual risk assessment is employed. Patient risk in terms of age, smoking, prior cancer history, occupational exposure to carcinogens, and the presence of hemoptysis are all weighed as well as the presence of any prior diagnosis of cancer. The pulmonary nodule evaluated by helical, preferably multidetector, CT is assessed for
nodule size, calcification, border regularity, density, and cavitation. The individual patient’s risk for tissue sampling and surgery is next weighed. This risk assessment requires significant mental calculus for the clinician, often facing discordant information (7). In general, the smaller the nodule, the younger the patient, the less the exposure to carcinogens, the greater the probability that the nodule is benign. However, risk of cancer often falls into an intermediate range, and a decision must be made as to whether to observe the lesion or to secure a histological diagnosis.
nodule size, calcification, border regularity, density, and cavitation. The individual patient’s risk for tissue sampling and surgery is next weighed. This risk assessment requires significant mental calculus for the clinician, often facing discordant information (7). In general, the smaller the nodule, the younger the patient, the less the exposure to carcinogens, the greater the probability that the nodule is benign. However, risk of cancer often falls into an intermediate range, and a decision must be made as to whether to observe the lesion or to secure a histological diagnosis.
A variety of tissue sampling techniques are available: fiberoptic bronchoscopy and biopsy, percutaneous needle aspiration or needle biopsy, video-assisted thoracoscopy, and thoracotomy. Bronchoscopy is least invasive and thoracotomy will yield the most certain histologic opinion. The morbidity and small, but real, risk of death increase with the invasiveness of the tissue sampling procedure. This risk of invasiveness correlates with increased certainty of the yield of biopsy from about 50% to nearly 100% (8,9,10). The clinician commonly weighs watchful waiting with serial CT scans every 3 months for 2 years versus a tissue sampling procedure and then deciding which procedure. Nodules near the periphery of the lung are better suited to needle biopsy, whereas nodules centrally located near the major airways are often better candidates for bronchoscopic biopsy. Nodules in the middle area of the lung are often quite hard to reach reliably by either methods. Since the nonsurgical approaches have sensitivities less than 100%, a negative study does not exclude the presence of cancer. Thus, some centers often try to move to the most definitive procedure quickly, especially if a negative result will not be sufficient to plan future management.
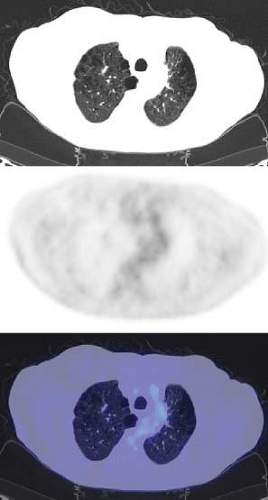
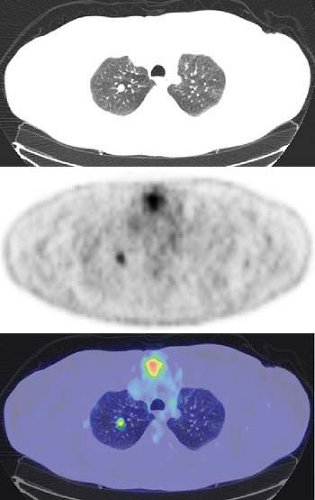
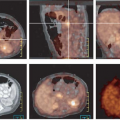
An FDG PET has proved to be quite sensitive for the detection and evaluation of pulmonary nodules. Gould et al. (11), in a meta-analysis of 40 published studies, demonstrated that the sensitivity of FDG PET was 96.8% and the specificity was 77.8% in 1,474 focal lesions, all greater than 1 cm. FDG PET compared to histology in these published studies was highly sensitive and moderately specific. They stressed the negative predictive value of 97.6% of FDG PET. This suggested that applied to patient populations with 1 cm or larger nodules similar in risk factors to those from the meta-analysis, a patient with a negative FDG PET would have a posttest likelihood of malignancy in the SPN of less than 3% (Fig. 8.9.1). One of the challenges, however, is that the risk is not reduced to 0%, so that some follow-up imaging remains necessary if a choice is made to observe a patient after a negative PET study. Another challenge is that physicians are often asked to perform PET scans in patients with solitary pulmonary nodules less than 1 cm in diameter. In such patients, the diagnostic sensitivity is probably lower due to recovery coefficient limitations of PET scanners and the scan becomes of greatest value when positive.
Many schemas combining PET and biopsy strategies have been proposed. Typically, PET and biopsy provide complementary information (12). In some approaches, tissue sampling is used for greatest certainty, and PET covers those patients where the nodule was inconvenient to biopsy or nondiagnostic. In other schema PET is performed first, and if PET is positive, patients may in some centers go straight to surgery. Even when PET is applied only when the biopsy was nondiagnostic, FDG PET performs well to diagnose lung cancer (13) (Fig. 8.9.2).
Gould et al. (7) used decision analysis to create a management strategy for the physician managing the deployment of the many available diagnostic options for pulmonary nodules. Their analysis found four major roles for PET in evaluating a pulmonary nodule: (a) when a patient’s risk of lung cancer and CT findings are not congruent, (b) when the surgical or procedure biopsy risk is high, (c) when a nondiagnostic biopsy occurs, or (d) when the patient is uncomfortable with watchful waiting. The sensitivity analysis demonstrated the broad utility of PET for evaluating pulmonary nodules.
Dynamic contrast enhancement with multidetector CT (MDCT) is more effective in categorizing SPNs as benign or malignant than noncontrast CT. A recent evaluation of integrated PET/ CT was compared to dynamic contrast MDCT. FDG PET/CT had a significant advantage in sensitivity (96% vs. 81%) with similar specificity (88% vs. 93%). PET/CT exhibited negative predictive values similar to established PET data, but with improved positive predictive value. In a head-to-head comparison of state-of-the-art
imaging technology, PET/CT provides greater accuracy than MDTC. These techniques may be complementary, but the use of contrast-enhanced CT for characterization of solitary pulmonary nodules does not appear to be as widely adopted as is PET/CT scanning, which seems appropriate based on the performance characteristics of the two methodologies (14).
imaging technology, PET/CT provides greater accuracy than MDTC. These techniques may be complementary, but the use of contrast-enhanced CT for characterization of solitary pulmonary nodules does not appear to be as widely adopted as is PET/CT scanning, which seems appropriate based on the performance characteristics of the two methodologies (14).
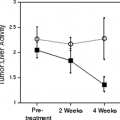
Integrated PET/CT compared to PET provides somewhat improved or similar nodule resolution, faster scanning times, and CT visualization of SPN. PET/CT technology provides additional methods of evaluating and improving evaluation of a pulmonary nodule. Dual point imaging involves FDG uptake measurements at two different times, typically separated by an hour. The SPN is evaluated by both its intensity of FDG uptake and the change over time. Most lung cancers have a high standardized uptake value (SUV) and the SUV increase is greater than 10% in the 1-hour period. Some, but not all, inflammatory processes have a decline in FDG uptake in this period of observation. However, some inflammatory processes can have rises in FDG uptake with time, and most centers do not currently apply the dual time point technique, in part due to logistical reasons (15). Although virtually all of the literature on PET and PET/CT with FDG has been developed using a 50- to 60-minute uptake period, there are some groups that perform the PET imaging at 90 minutes or more post-FDG injection. Although tumor/background ratios typically rise in this period of time, some caution must be used in interpretation, as interpretation criteria were developed based on the 1-hour uptake period and may differ slightly with a 90-minute uptake period.
The apparent FDG uptake of a nodule is impacted by the partial value effect. The size of the SPN on CT can be used to correct for partial volume effect. Initial research suggests that partial volume correction improves SUV measurements of nodules smaller than 2 cm and increases the sensitivity for malignancy (16). Movement of a SPN with respiration during a PET/CT study causes the counts from the FDG uptake to be distributed over multiple pixels. However, for small nodules, the correction in the SUV is substantial and dependent on lesion size. Measurements of lesion size for small lesions are quite subject to variability, and this variability can be propagated to the SUV assessments. Thus, many interpreting physicians perform a “visual” partial volume correction, in which they use their clinical judgment to realize that modest levels of FDG uptake, sometimes no more than blood pool, in lesions under 1 cm, may be indicative of the presence of a small cancer. Respiratory gating, especially in the lower lobes, corrects for the respiratory excursion of 1 to 3 cm during PET imaging. The apparent activity within the moving nodule may increase between 20% and 36% (17). These techniques show promise but need further investigation before full utilization is likely. It must be realized that some pulmonary nodules that are malignant or behave in a malignant fashion may have relatively low SUVs. Bronchioalveolar carcinoma is very commonly in this category and in the pure state they have SUV values that are much lower than other cancers, sometimes less than 2.5. Carcinoid tumors, both primary and metastatic, can also have low SUVs relative to more typical lung cancers. The CT characteristics of bronchioalveolar carcinoma often are quite suggestive of the presence of this tumor, even if the SUV is low.
When interpreting PET in SPN, a variety of interpretation schemes can be used. Since most PET imaging involves the use of PET/CT, it is important that the CT be examined to determine lesion size. Smaller lesions, less than two times the resolution of the imaging system, will not have all counts recovered, so their apparent SUV will be lower than their true radioactivity concentration. In many centers, a qualitative interpretation is applied in which SPNs with FDG uptake greater than that in blood pool background are called “positive” while those with less than blood pool background are called “negative.” Quantitative cutoff values of SUV have been proposed with 2.5 as a good separator of malignant and benign nodules; however, in practice, measurement errors could make a lesion with a true SUV of 2.7 appear to have an SUV of 2.4 (due to variance of the measurement method). Most centers tend to err on the side of sensitivity as opposed to specificity in interpreting PET of SPN to avoid false-negative results. A review of the meta-analysis of Gould et al. (7) shows that although a joint operating sensitivity/
specificity of 91% is possible with PET using FDG in SPN, most readers operate to achieve higher sensitivity at the tradeoff of lower specificity.
specificity of 91% is possible with PET using FDG in SPN, most readers operate to achieve higher sensitivity at the tradeoff of lower specificity.
Non–Small Cell Lung Cancer
Non–small cell lung cancer (NSCLC) comprises about 80% of all lung cancers, and many patients are smokers or have had significant exposure to secondhand smoke. The most common cell types, in order of occurrence, are adenocarcinoma, including bronchoalveolar variants, squamous cell carcinoma, and large cell. In the past decade, the frequency of adenocarcinomas has grown steadily. Some of the patients with adenocarcinomas are nonsmokers, and a significant fraction are females (18). Surgical resection represents the best opportunity for cure, but recently combination therapy including chemotherapy, radiation therapy, and to some extent biological therapy has been employed with increasing frequency. NSCLC staging is based on the TNM system and requires accurate characterization of the primary tumor (T), regional lymph nodes (N), and distant metastases (M). Staging is important for appropriate selection of treatment and assessment of prognosis.
Non–small Cell Lung Cancer Tumor Stage
Tumors are described as T1 when they are 3 cm or less in greatest dimension arising distal to a main bronchus. A T2 tumor is one that is larger than 3 cm, invades the visceral pleura, or has local atelectasis or obstruction associated with it and is located greater than 2 cm from the carina. A T3 tumor invades the chest wall, diaphragm, mediastinal pleura, or pericardium or is associated with diffuse atelectasis or obstruction, and it can be 0 to 2 cm from the carina without involving the carina. A T4 tumor is one that invades vital mediastinal structures such as heart, great vessels, trachea, carina, or vertebral bodies or has a malignant effusion or separate tumor nodules in the same lobe. These stages are largely intended to distinguish which tumors are and are not resectable—all but T4 being potentially resectable. These tumor stages are components of the TNM staging classification (19).
Traditionally T stage information has been obtained by CT and depends on anatomic abnormalities detectable by CT. This can be most helpful when determining the direct extension and involvement of the tumor into normal structures that can change disease stage. FDG PET alone had played a minor role in T staging due to challenges in measuring tumor size and in determining the precise borders/extent of the FDG uptake. Integrated PET/CT provides improved T staging in the detection of chest wall and mediastinal invasion and for evaluating the degree of atelectasis involved in the CT tumor mass (20). The detection of malignant pleural effusions and pleural metastases is substantially aided by PET/CT evaluation.
Non–small Cell Lung Cancer Node Stage
The presence of metastatic disease in lymph nodes correlates closely with worsening survival. N0 status indicates no nodal metastases. An N1 status implies a metastasis to ipsilateral peribronchial, lobar, or interlobar nodes. N2 implies a metastasis to ipsilateral mediastinal or subcarinal nodes. N3 indicates involvement of contralateral lymph nodes, scalene, or supraclavicular nodes. N0 status correlates with a 56.5% 5-year survival rate, and N1 disease correlates with a 47.5% 5-year survival rate. Only a 10% to 20% survival rate or less is seen once N2-level nodes are involved. Generally, those with N3 lymph node disease do not survive 5 years, and N3 disease is commonly a contraindication to surgery (21).
The noninvasive assessment of mediastinal nodes for tumor involvement is relatively difficult with CT. Because of the possibility of nodal enlargement from reactive or inflammatory lung diseases or even congestive heart disease, there is ample probability that enlarged nodes may not be involved with tumor. The nodal size limit of normality used in CT imaging is about 1 cm for the short axis of the lymph node. Therefore, any lymph node containing tumor that is less than 1 cm in short axis length will not be called abnormal by size criteria. Any lymph nodes that are larger than 1 cm will be called abnormal and are suggestive of a metastasis in a patient with a lung cancer history. Many such enlarged nodes do not contain malignant cells but are enlarged due to other causes.
Alternatively, invasive sampling with mediastinoscopy can be performed to assess lymph node status. This procedure is unable to assess all lymph nodes with a single point of access. Specifically the aortopulmonary lymph nodes cannot be assessed without a different approach to the lymph nodes. The result is that the selection of lymph nodes for biopsy by the mediastinoscopy technique is based on accessibility. This results in a high, albeit imperfect, sensitivity for mediastinal disease of about 90% (22,23). It is unlikely that this will improve due to the limitations of the technique. Transbronchial needle biopsy of lymph nodes is also useful but is limited in sensitivity in part due to its “blind” nature and due to areas that cannot be sampled. This approach can be guided by PET and by ultrasound, but still suffers from sampling errors that lower sensitivity of the method.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree