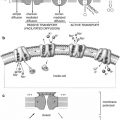
Class
Causes
Primary
Congenital lymphedema
Praecox (before age 35)
Tarda (after age 35)
Secondary
Inflammation
Malignancy
Radiation therapy
Filariasis
Surgical dissection
Trauma
Recurrent dermatitis
19.3.2 Lymph Nodes with Metastases
In general, tumors can metastasize by several routes including venous, arterial, lymphatic, and local invasion. It is believed that while most tumors initially spread through the lymphatic system, temporarily being retained at successive levels of lymph nodes by the body’s defense system, some tumors may spread through both the vascular and lymphatic systems nearly simultaneously. Since lymph nodes are common sites of metastasis, knowledge of their involvement is crucial for patient management and prognosis. When small numbers of tumor cells (micrometastases) are found in lymph nodes, the architecture and physiological characteristics of the lymph node are not altered. Even with larger tumor loads, lymph nodes may remain normal in size, making them difficult to detect with anatomical imaging studies. Determination of focal defects within lymph nodes secondary to tumor infiltration is usually unreliable with all current imaging modalities [8].
19.4 Nuclear Medicine Applications
19.4.1 Basic Principles of Nuclear Medicine Imaging
The tracer is injected into a specific location, and imaging is then performed while the material crosses into the lymphatic system and migrates toward the vascular space. Agent movement will depend on the specific radiopharmaceutical used, the particle size, and the location of the injection. Particulate agents such as colloids are not transported into the peripheral collection sites as well because of their larger size. However, they are better retained in the lymph nodes because of their localization within RES cells. Nonparticulate agents travel much faster and efficiently but are not retained within a lymph node because they do not localize to any of the tissue components but are simply passing through.
The optimal size of colloids for lymphoscintigraphy is approximately 50–70 nm [9]. Particles smaller than few nanometers usually leak into the blood circulation. On the other hand, particles larger than 100 nm usually get trapped into the interstitial compartment for a relatively long time [10].
Multiple radioisotopes have been used to perform lymphoscintigraphy. The common clinically used Tc-99m-labeled isotopes include antimony trisulfide colloid, sulfur colloid, albumin colloid, and human serum albumin (HAS). Sulfur colloid has a relatively large particle size (30–1,000 nm) and therefore has minimal absorption and slow transport from the injection site. To reduce the particle size, filtered sulfur colloid has been used with approximate particle size of less than 100 nm. Antimony trisulfide colloid has similar properties to filtered sulfur colloid. Albumin micro-colloid has a small particle size (<80 nm) and shows rapid clearance from the injection site. It has easy labeling properties and is more suitable for quantitative studies [10].
Because of the very rich supply of lymphatics in the skin, injections into this location will show very efficient uptake and movement of tracer. Multiple injection techniques have been used clinically including subcutaneous, intradermal, and subfascial (intramuscular) injections. Some authors believe that subcutaneous injections are more reliable because intradermal injections result in tracer uptake by blood vessels [11].
19.4.2 Detection and Follow-Up of Lymphedema
Lymphoscintigraphy can demonstrate (a) clearance of radiolabeled colloid from an interstitial injection and (b) flow to regional lymph node(s), along with some lymph node anatomical features. Several acquisition protocols can be used. Most investigators have found this test to be sensitive but not specific for determining the etiology of a patient’s edema. The procedure usually consists of repeated acquisitions over the upper or lower limbs, depending on the site of the edema, to demonstrate the movement of the depot through the lymphatics to the axillary or inguinal lymph nodes, respectively. Early (15–30 min) images are compared against delayed 2–6 h images for travel of the radiotracer through lymphatics and appearance of lymph nodes. Exercise improves visualization of lymphatics and is encouraged in between imaging. The interpretation depends on visual assessment of the injection sites, lymphatic tracts, and lymph nodes.
A 45-min dynamic acquisition over the axilla/groin and repeated spot views over the injection site over several points of time both can be utilized to derive a semiquantitative parameter. These include time-activity curves for timing of tracer appearance in lymph nodes, tracer washout from injection site, and tracer appearance and washout in lymph nodes. These parameters are highly dependent on the type of the radiopharmaceutical, injection technique, and exercise. Therefore, care is advised when using such measures [7].
19.4.2.1 Normal Scintigraphic Pattern
In the lower limbs, normally, there is rapid and fairly symmetrical transport of the radiotracer from foot injections through one or two lymphatic vessels in the calf and one lymphatic vessel in the thigh. Multiple pelvic lymph nodes should be clearly visualized within 1 h. Time-activity curves generated with a region of interest over the inguinal lymph nodes in lower-extremity studies and over axillary nodes in upper-extremity studies normally show a steady rise of activity, especially if a particulate agent is used. Considerable activity usually remains at the injection site throughout the study, with approximately only 40 % having migrated by 90 min.
19.4.2.2 Scintigraphic Patterns of Lymphedema
Scan findings in patients with lymphedema will depend on the cause of the swelling, the length of time that the process has been present, and compensatory mechanisms that have developed to circumvent the flow disturbance [10]. Figure 19.1 shows the pathological mechanisms that lead to lymphedema and the corresponding scan patterns and Figure 19.2 represents a lymphedema study. Nawaz et al. described three abnormal patterns for lower-extremity lymphedema: a pattern of stasis, an obstructed pattern, and an enhanced pattern [12]. Lymphatic obstruction will initially cause proximal vessel dilatation and delayed flow, followed by dermal back diffusion with dispersion of activity into the soft tissues where the tracer originated, along with the development of collateral lymphatic channels. Rapid flow from the injection site is the hallmark of the enhanced pattern, with fast visualization of draining lymph nodes [11].
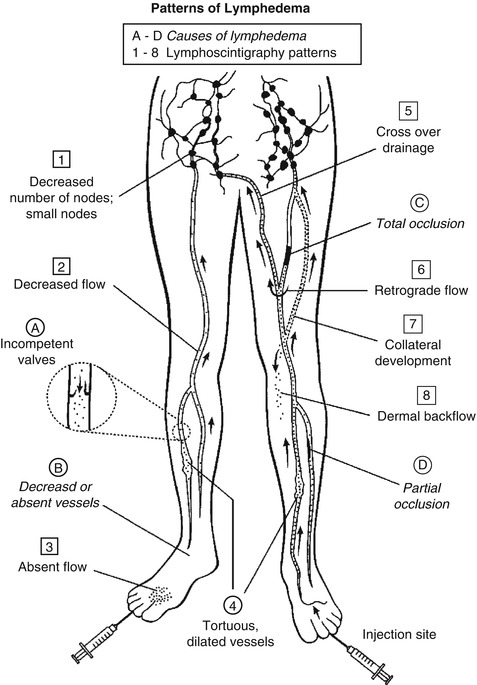

Fig. 19.1
Pictorial representation of the causes of lymphedema and the scan patterns that are created
19.4.3 Detection of Lymph Node Metastases
Since lymph nodes have reticuloendothelial cells that phagocytose foreign material, radiocolloids are used to visualize them. Direct determination of the presence of tumor is extremely difficult, since the desired space-occupying defects caused by tumor infiltration require a significant portion of the node to be involved. When lymphatic tissue is largely replaced by tumor, lymph nodes may not be visualized because the tracer is blocked from entering. Tumor-involved nodes can even show more tracer uptake than normal nodes [13]. This may be explained by reactive changes in the lymph node, with increased numbers of RES cells being present, possibly in reaction to the presence of tumor antigens.
Lymphoscintigraphy with radiolabeled antitumor antibodies such as anti-CEA has been used to detect occult tumor in lymph nodes. Contrary to radiocolloid lymphoscintigraphy, which depends on phagocytosis, radiolabeled antibody localization requires attachment of the antibody directly to tumor cells. Interstitial injection of these agents has the advantage of producing a higher concentration of tracer at the tumor site in the lymph node than when the antibody is injected intravenously. However, the presence of a definitive number of metastatic cells is required for detection, depending on the agent and the imaging technique used. More recently PET-FDG is being used to detect more effectively lymph node metastasis of many tumors. It has been shown to be particularly useful in detecting lymph node metastasis of lung cancer changing the mode of therapy in a significant number of cases [14].
19.4.4 Sentinel Node Detection
19.4.4.1 Concept
The concept of sentinel lymph node (SLN) was first introduced by Cabanas in penile carcinoma in 1977 [15]. The lymph node(s) that receives initial lymphatic drainage from a location harboring tumor has been termed the “sentinel node.” Tumor cells gain access to the lymphatic system initially through peritumoral lymphatics facilitated by the lack of basement membrane and presence of large gap junctions between endothelial cells lining the channels. The aggressiveness of the tumor and the abundance of lymph vessels are important factors in lymphatic invasion. There is an orderly sequence of tumor emboli dissemination from the primary site first to the SLN and thereafter to remaining non-SLN in the basin prior to other systemic sites. There can be single or multiple sentinel nodes which may be located in one or different lymphatic beds [13]. Since determination of lymph node involvement is an integral part of tumor staging and management, lymph node excision with pathological evaluation is commonly performed. A complete nodal dissection (often involving large areas of tissue), however, can cause considerable morbidity, including lymphedema, and still fail to remove small diseased nodes [16] or nodes in a different draining bed. If the sentinel node(s) can be identified, extensive pathological examination of a single or few nodes can forecast whether tumor dissemination has occurred, since it is the first filter that metastatic cells encounter. Sentinel nodes can be identified by blue dye injection around the tumor just before surgery or by using a radiopharmaceutical injected in a similar fashion [17–19]. The combination of the two techniques leads to higher identification rate of the sentinel node in most cases. Radioactive sentinel nodes can be detected using preoperative imaging with a gamma camera or intraoperative gamma probe. Lymphoscintigraphy using dynamic and static imaging better defines the sequence of lymphatic flow from the tumor site to draining lymph nodes, especially the sentinel node.
There are two important parameters to measure the utility of the SLN, namely, identification rate and the false-negative rate. The SLN identification rate is the proportion of successful procedures in which the SLN is located and removed. Failure to identify the SLN, though unfortunate, leads to exploration of lymph nodes, so the consequence is that the patient will not be spared an extensive surgery. In order to correctly determine the false-negative rate, SLN biopsy should be carried out in a validation study where all patients should receive standard lymphadenectomy, and so in this context, the false-negative SLN biopsy is defined as presence of metastasis in a non-SLN. The consequence of false-negative SLN is devastating since the patient is improperly downstaged, does not receive definitive therapy, and possibly have relapse and higher mortality [20]. Therefore, the most important factor in SLN biopsy is the false-negative rate [21].
19.4.4.2 Radioisotopes for SLN Lymphoscintigraphy
The rate of tracer uptake in lymph vessels and consequent filtration in a sentinel node is governed by the particle size. The optimum size is estimated 5–10 nm as smaller particles may gain access to the vascular system. Filtration of the colloid allows control of the particle size to 15–50 nm, while unfiltered nanocolloids have a wide range of particle size from 5 to 1,000 nm [22].
Usually, 0.5–1 mCi of Tc-99 m sulfur colloid or nanocolloid is injected in divided dose in the tumor, around the tumor, or in the overlying skin or mucosa. The transit time to the lymph node is variable, depending on the location, but is generally less than 1 h, and activity may be retained in the lymph node for further 3–6 h. The injection, dynamic or static scintigraphy, and intraoperative gamma probe localization are completed on the same day, although the latter can be carried out after an afternoon injection and imaging on the preceding day for logistic purposes.
19.4.4.3 Tumors
Breast Cancer
The breast has three main lymphatic pathways: internal mammary, axillary, and supraclavicular/infraclavicular [23]. Drainage into the associated groups of nodes occurs from cutaneous, subcutaneous, or parenchymal collection sites. Despite the individual variability in drainage pattern, most individuals have drainage into the axillary lymph nodes from all locations in the breast, with only about 9 % having regions that drain exclusively into the internal mammary chain [24, 25]. Most of the breast sites that drain into the internal mammary chain are on the medial aspect of the breast, but they can be located elsewhere. Drainage from one internal mammary chain to the contralateral chain is common. Sentinel nodes could also be located within the breast itself [26]. Direct drainage to other nonaxillary sites occurs but is even less frequently encountered. Axillary nodes are divided surgically into three groups or levels: I, II, and III, in relation to the pectoral muscle (Fig. 19.2). In the past this was presumed to represent the sequence in which lymph nodes become involved with metastatic cells from primary breast tumors.
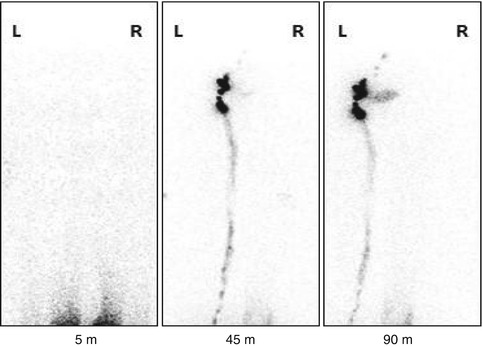

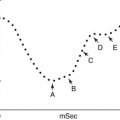
Fig. 19.2
Lymphoscintigraphy study of a patient with right lower limb edema.The 5 minute image shows beginning of ascent of the radiotracer from the injection sites. The 45 and 90 minute images shown illustrates normal drainage of the radionuclide in the left side with visualization of inguinal lymph nodes (arrow) and lymph channels (arrow head) and lack of migration in the right side
Involvement of axillary lymph nodes is an important prognostic factor in breast cancer. Axillary lymph node dissection has long been the standard practice in breast surgery for staging, chemotherapy recommendation, and local disease control. A typical dissection consists of removal of both level I and level II lymph nodes for pathological evaluation (Fig. 19.3). Level III nodes are not routinely excised because of the increased incidence of postoperative lymphedema in the adjacent upper extremity [27]. Because axillary nodes are numerous, vary greatly in both number and location, and can be buried deep in fatty tissue, a complete resection is difficult. Lymph nodes harboring disease may therefore be missed because of sampling error. This may partially explain why 25 % of patients with initially negative axillary nodes subsequently develop recurrent disease [25]. Alternatively, removal of uninvolved nodes potentially eliminates a filter that may be able to slow further tumor migration. Finally, morbidity from a complete axillary dissection can be significant and includes the postoperative development of lymphedema, seromas, neuromas, paresthesia, problems with wound healing, and a decrease in adjacent arm motion which affect the patient’s quality of life. A paradigm shift in the surgical approach of breast cancer was the introduction of sentinel lymph node dissection, where a sentinel node status reflects the status of the entire lymph node basin. Patients with clinically negative axillary nodes (based on clinical examination, imaging, or FNA) would undergo SLN biopsy at the time of the breast surgery. Accordingly, only patients with a positive SLN would proceed to axillary lymph node dissection, and those with a negative SLN are spared the procedure. The overall survival, disease-free survival, and regional control of node-negative patients undercounting either axillary dissection or SLN biopsy are equivalent [28]. More recent data suggests that in selected patients with a positive SLN, axillary dissection may be spared in patients undergoing breast conserving surgery and whole-breast irradiation [29].
The typical procedure includes injecting Tc-99 m nanocolloid or Tc-99 m sulfur colloid (filtered or unfiltered) interstitially around the tumor at one to four locations. Ultrasonography can be used to help localize the tumor when needed. The radiotracer is injected along with saline intradermally around the tumor. Some investigators use injections around the areola. The volume used varies from 0.2 to 0.8 ml per injection site. Imaging is obtained in the morning of surgery for up to 2 h and is followed by intraoperative probe localization. Alternatively, imaging can be obtained at the end of the day, with surgery and probe localization the following morning. Images are obtained usually in the anterior oblique projections. Static images with transmission and markers are also obtained in the anterior oblique and optionally anterior and lateral projections according to the location of the visualized node(s) (Fig. 19.4). Dynamic imaging over the breast and axilla is helpful to differentiate a sentinel lymph node from next-echelon nodes [30]. The addition of SPECT/CT imaging improves anatomical localization and so provides better outline for subsequent surgical approach. Overlapping, deep-seated, and outside-the-axilla nodes (including intramammary, internal mammary, and interpectoral) are easier to resolve on SPECT/CT [31, 32].
Intraoperative gamma probe localization can be used with or without blue dye injection to localize the SLN as a hot (and/or blue) node. The success of the biopsy is confirmed by ex vivo counting of the node and decreased counts in the axillary bed.
The SLN identification rate is reported to be 90 % and a false-negative rate of 12 % in a meta-analysis of 21 studies [33]. A limited number of cases of inflammatory breast cancer exhibited a lower identification rate [34].
The detailed examination of fewer lymph nodes by a pathologist translates into the ability to detect smaller volume of metastasis, and so micrometastasis and even isolated tumor cells could be identified. The clinical utility of micrometastasis detection may involve the decision making regarding adjuvant therapy; however, there is no enough data to support the necessity for further axillary node dissection [35].
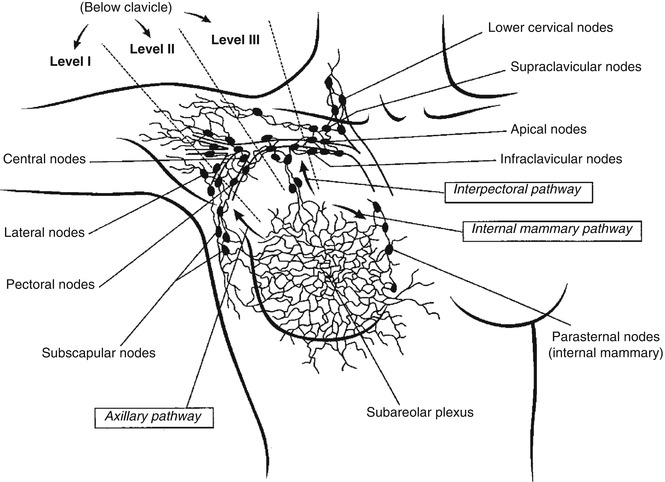
Fig. 19.3
Breast lymphatic tracts, nodes, and their surgical classification (From Krasnow and Hellman [26], with permission)
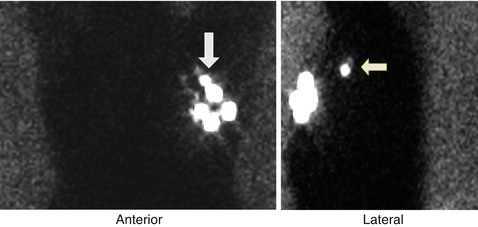
Fig. 19.4
A sentinel lymph node localization study in a patient with left breast cancer showing visualization of a sentinel lymph node in the anterior projection and more clearly in the left lateral projection (arrows)
Melanoma
Malignant melanoma originates from melanocytes (melanin-producing cells) and currently has an alarming increase in incidence among all types of cancer [36]. Primary neoplasms are usually found in the skin but can also develop in melanocytes of the eye or bowel. Factors implicated in the pathogenesis of the tumor are:
Genetic predisposition
Exposure to ultraviolet light
Fair hair
Light skin
Steroid hormone activity
Freckles
Elevated steroid hormone levels have been linked to the development of melanoma since it can initiate the pigmentation process in unpigmented nevi. The tumor arises from malignant degeneration of melanocytes in the basal layer of the epidermis or in a melanocytic nevus consisting of an aggregation of melanocytes.
The biology of this tumor is complex but there are four major types of melanoma:
1.
Superficial spreading melanoma is an intraepidermal horizontal macule that slowly grows into a plague.
2.
Nodular melanoma is a nodular, often bleeding, lesion with aggressive vertical growth and short or absent horizontal growth phase.
3.




Lentigo maligna melanoma arises from melanoma in situ (lentigo maligna) mainly in sun-damaged skin of elderly patients and is characterized by presence of atypical melanocytes at dermoepidermal junction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree