Fig. 16.1
Diagram of the relevant parts of the digestive system
16.1 The Esophagus
16.1.1 Anatomic and Physiological Considerations
The esophagus is a 25 cm-long muscular tube that passes through the mediastinum, connecting the pharynx and the stomach. The cervical esophagus is composed of striated muscles whereas the thoracic esophagus consists of smooth muscles. Physiologically, the main function of the esophagus is to transport swallowed food from the hypopharynx to the stomach. Moreover, the normal function of the esophagus prevents the regurgitation of food from the upper esophagus into the hypopharynx and gastric contents from the stomach to the esophagus. The disruption of the normal function of the esophagus results in dysphagia and/or regurgitation. Functionally, the esophagus has three components: the upper esophageal sphincter (UES), the body, and the lower esophageal sphincter (LES). The components work together to keep the esophagus empty. Esophageal motility disorders result from sphincter dysfunction or abnormal peristalsis in the body of the esophagus or both. The diagnosis and treatment of esophageal dysmotility rest on the understanding of the functional anatomy of the UES, esophageal body, and the LES.
16.1.1.1 Upper Esophageal Sphincter
The UES is a 3–4 cm-long high-pressure zone that forms a barrier between the esophagus and the pharynx. It opens and closes intermittently to allow the passage of food or liquid. The UES closure muscles comprise the cervical esophagus, cricopharyngeus, and interior pharyngeal constrictor. The UES opening muscles consist of the superior and inferior hypoid muscles and superior pharyngeal muscles [1]. All UES muscles are striated and are innervated by the glossopharyngeal, branches of the vagus, ansa cervicalis, and sympathetic nerves from the cervical ganglion. The vagus nerve is the major motor nerve of the UES. Nerve cell bodies of the vagus efferent fibers are located in the nucleus ambiguus (medulla oblongata). Between swallows, the UES is tonically contracted to prevent reflux of esophageal contents and entry of air from the pharynx during inspiration. During swallowing, the cricopharyngeal and inferior pharyngeal constrictor muscles relax and the suprahyoid muscles contract.
16.1.1.2 Esophageal Body
The esophageal body extends from the UES to the LES and measures 18–24 cm. The esophageal wall consists of mucosa, submucosa, and the tunica muscularis and adventitia. The esophageal body is not surrounded by a tunica serosa. The esophageal mucosa is of the stratified squamous epithelium type, except for the distal 2 cm, where columnar epithelium of the gastric cardia type may be encountered. More proximal extension of gastric-type epithelium or the presence of intestinal-type columnar epithelium defines the pathological entity known as Barrett’s esophagus (Fig. 16.2). Outside the epithelial lining is a thin layer of longitudinally oriented smooth muscle fibers, the muscularis mucosa. Below the muscularis mucosa is the submucosa, which consists of connective tissue. The muscularis propria is made up of an inner, circular, muscle layer and an outer, longitudinal, muscle layer. In the proximal 5 % of the esophageal body, the muscularis propria is made up of striated muscle fibers. The distal 50–60 % consists entirely of smooth muscle. The middle 35–40 % is composed of a mixture of smooth and striated muscle fibers.
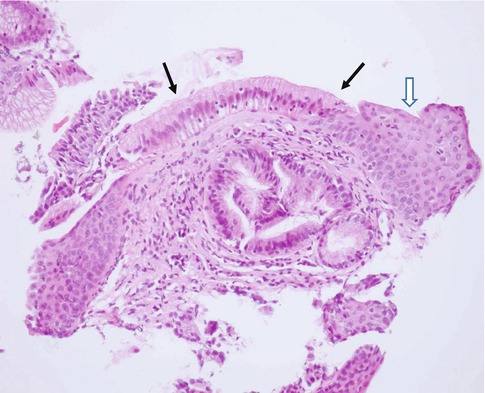

Fig. 16.2
Barrett’s esophagus. Solid arrows point to columnar metaplasia. Open arrow points to the normal stratified squamous epithelium (Courtesy of Prof. M. Elmonayeri)
Neuronal control of esophageal body motility is complex [2]. The esophageal wall receives extrinsic innervation via the vagus nerve. Striated muscle fibers are directly innervated by postganglionic neurons originating in the nucleus ambiguus and terminating on the motor end plate. Smooth muscle fibers are controlled by preganglionic nerve fibers originating in the dorsal motor nucleus. These cholinergic nerve fibers terminate on the intrinsic neurons of the myenteric plexus located between the circular and longitudinal muscle layers. Within the myenteric plexus, two types of neurons have been identified. Excitatory neurons mediate contraction of both longitudinal and circular muscle layers via nicotinic cholinergic nerve receptors. Inhibitory neurons mediate the relaxation of mainly circular muscle fibers via noncholinergic, nonadrenergic neurotransmitters, most probably nitrous oxide and vasoactive intestinal peptide (VIP) [3]. Intrinsic sensory (afferent) neurons are within Meissner’s plexus located in the submucosa. Sensory impulses are conveyed to the central nervous system via both vagal and thoracic sympathetic nerve fibers.
Swallowing initiates a progressive series of coordinated propulsive contractions throughout both the striated and the smooth muscle portions of the esophageal body. This form of esophageal motor activity is referred to as primary peristalsis. Intraluminal distention of the esophageal body results in a peristaltic wave at or proximal to the site of distention. This wave is termed secondary peristalsis and serves to clear the esophagus from contents that have not been cleared by primary peristalsis, or refluxed gastric contents. Primary and secondary peristaltic waves have similar amplitudes and travel at a velocity of 3–5 cm/s.
Deglutitory inhibition is a unique physiological phenomenon whereby repetitive swallowing inhibits all esophageal body activity while the LES is relaxed. A normal peristaltic contraction will follow the last swallow of such a series and clear the esophagus completely [4].
16.1.1.3 Lower Esophageal Sphincter
The LES is a high-pressure zone measuring 2–5 cm in length located between the esophageal body and the stomach. Ultrastructural studies show that this high-pressure zone consists of a specialized thickened region of the circular muscles layer of the distal esophagus, albeit the muscle fibers are not circular, rather they are organized as clasp and sling fibers [5]. At rest the sphincter is tonically contracted with a normal pressure ranging from 10 to 45 mmHg. The basal LES tones are determined by three factors: myogenic tone that is independent of neural influences, cholinergic excitatory tone, and nitrergic inhibitory tone. The LES relaxes after swallowing. Relaxation is mediated by the nitrergic inhibitory neurons. The LES may also relax without swallowing, a phenomenon referred to as transient lower esophageal sphincter relaxation (TLESR). These relaxations are believed to play a major role in the pathogenesis of gastroesophageal reflux disease.
A number of endogenous compounds affect the LES tone when administered in pharmacological doses. For instance, large doses of gastrin increase the tone of LES. Exogenous substances such as beta-adrenergic receptor agonists and calcium channel blockers may induce relaxation of the LES.
16.1.2 Esophageal Motor Disorders
16.1.2.1 Disorders of the UES and Cervical Esophagus
Motor disorders affecting the skeletal (proximal) part of the esophagus result from either neurological abnormalities affecting the extrinsic innervation of the proximal esophagus, or skeletal muscle or neuromuscular disorders. More specifically these include:
Neurological diseases
Cerebrovascular accident
Parkinsonism
Amyotrophic lateral sclerosis
Cranial nerve palsy
Skeletal muscular disorders
Dermatomyositis
Polymyositis
Muscular dystrophy
Cricopharyngeus dysfunction
Others
Myasthenia gravis
Amyloidosis
Because of the difficulty of transferring food bolus from the hypopharynx into the esophageal body across the UES, most patients experience choking or regurgitation of liquids and/or solids. Videofluoroscopy is the best diagnostic modality for diagnosing oropharyngeal dysphagia. Scintigraphy is of limited value.
16.1.2.2 Disorders of Esophageal Body and LES
Disorders of the distal esophageal body (smooth muscle) and LES can be broadly classified into achalasia and nonspecific esophageal dysmotility according to conventional stationary esophageal manometry (Table 16.1).
Table 16.1
Classification of primary esophageal disorders
Achalasia |
Nonspecific esophageal dysmotility |
Hypercontractile esophagus |
Nutcracker esophagus |
Hypertensive lower esophageal sphincter |
Hypocontractile esophagus |
Ineffective esophageal motility |
Hypotensive lower esophageal sphincter |
Discoordinated motility |
Diffuse esophageal spasm |
Achalasia
Achalasia is the best-studied motor disorder of the esophagus. It occurs at a rate of 1:100,000 and affects both sexes equally. Age of onset is usually 25–65 years. It results from the degeneration of the inhibitory myenteric neurons in the body and the LES region. This leads to a hypertensive LES which relaxes poorly and also causes aperistalsis in the body of the esophagus. Patients usually present with dysphagia to liquids and solids. Barium esophagogram may show dilatation of the esophagus and “bird beaking” of the distal esophagus. Esophageal manometry confirms the diagnosis of achalasia. Typically, there is incomplete relaxation of the LES and aperistalsis. LES hypertension is detected in 20–40 % of patients [6]. Although these changes are highly suggestive of achalasia, they are by no means pathognomonic. Other conditions that might mimic achalasia include adenocarcinoma of the cardia, esophageal squamous cell carcinoma, Chagas’ disease, and lung cancer.
Several other spastic disorders have been characterized in patients with noncardiac chest pain (Table 16.1). They all share a similar clinical presentation. Diffuse esophageal spasm (DES) is the most severe form. It is less common than achalasia. The mean age at presentation is 40 years. Patients frequently complain of intermittent nonprogressive dysphagia for solids and liquids. Hot or cold liquids and stress may precipitate chest pain. Some patients with DES progress to achalasia.
Nonspecific Esophageal Dysmotility
Nonspecific esophageal motility disorders are further classified into hypercontractile, hypocontractile, and discoordinated motility. Patients with hypercontractile esophagus usually present with chest pain and dysphagia. Nutcracker esophagus is a hypercontractile disorder characterized by increased lower esophageal peristalsis amplitude and duration. Hypertensive LES is associated with an LES resting pressure of >45 mmHg [7, 8].
Patients with hypocontractile esophageal disorders complain of heartburn, regurgitation, and occasional dysphagia. Ineffective esophageal motility is characterized by low-amplitude peristalsis. Hypotensive LES is defined as an LES pressure below 10 mmHg. A number of systemic conditions are associated with esophageal hypocontractility including scleroderma, diabetes mellitus, and amyloidosis.
Diffuse esophageal spasm is a rare condition that presents with atypical chest pain that can radiate to the throat and back. Dysfunction in nitrous oxide synthesis or degradation is believed to be the cause of the spastic component. The manometric hallmark of diffuse esophageal spasm is simultaneous discoordinated contractions in more than 20 % of swallow. The resultant tertiary contractions give the corkscrew appearance on barium swallow.
16.1.2.3 Gastroesophageal Reflux Disease
Gastroesophageal reflux disease (GERD) involves the reflux of chyme from the stomach to the esophagus. The LES may relax spontaneously and transiently 1–2 h after the patient has eaten, allowing gastric contents to regurgitate into the esophagus. The acid is normally neutralized and cleared by peristalsis from the esophagus within 3 min and the tone of the sphincter is stored. When the reflux does not cause symptoms, it is known as physiological but in some individuals it may cause a spectrum of inflammatory responses in the esophagus. GERD is the most prevalent condition originating from the gastrointestinal tract. It is estimated than 20 % of the Western adult population suffers from heartburn more than three times a month [9]. It is particularly important in the pediatric age group. Also, GERD is common among pregnant women, especially during the third trimester.
The typical symptom of GERD is heartburn. However, a number of atypical symptoms are also linked to GERD, such as noncardiac chest pain, hoarseness, asthma, water brash, teeth erosion, and halitosis.
Most children affected with gastroesophageal reflux (GER) are between 6 months and 2 years old; they suffer from poor weight gain, vomiting, aspiration, choking, asthmatic episodes, stridor, apnea, and failure to thrive. A small amount of physiological reflux occurs in infants and resolves spontaneously by 8 months of age. The Tuttle acid reflux test is generally considered the reference standard but is technically demanding. The radionuclide method has a number of potential advantages. It is physiological, easily performed, well tolerated by the patient, and quantitative and involves a low radiation dose to the child.
Pathophysiology
GERD is a multifactorial process. Causes of GERD can be categorized as follows: (a) decreased pressure of LES, (b) transient increase in intra-abdominal pressure, and (c) short intra-abdominal esophageal segment. Mechanisms involved are summarized in Table 16.2. As mentioned earlier, transient LES relaxation appears to be the most common mechanism of GERD, especially in patients without endoscopic evidence of esophagitis [10]. In patients with moderate to severe esophagitis, LES incompetence plays a more important role in promoting reflux. The relation between a sliding hiatus hernia and GERD is controversial. Although most patients with severe GERD have hiatus hernia, most patients with hiatus hernia are asymptomatic. Recent data suggest that a large hiatus hernia may impair acid clearance [11, 12].
Table 16.2
Mechanisms of gastroesophageal reflux disease
Mechanism | Causes |
|---|---|
Anti-reflux barrier | Transient LES relaxation |
Incompetent LES | |
Sliding hiatus hernia | |
Esophageal clearance | Impaired peristalsis |
Decreased salivary output | |
Refluxate composition | Acid |
Pepsin | |
Bile salts | |
Pancreatic enzymes | |
Gastric factors | Delayed gastric emptying |
Acid hypersecretion | |
Helicobacter pylori | |
Defective esophageal mucosal protection | Lack of HCO3 secretion |
Lack of mucus secretion |
Esophageal body peristalsis plays an important role in clearing refluxed acid in both the upright and the supine position. Defective primary or secondary peristalsis leads to incomplete clearance of acid. Furthermore, salivary HCO3 usually neutralizes acid that remains in contact with the esophageal mucosa. Thus, impaired salivation may contribute to mucosal injury [13].
There is consensus about the fact that the potency of the contents of the refluxate, particularly acid/pepsin, is important in the pathogenesis of reflux esophagitis. Bile and pancreatic enzymes may be additional contributing factors.
Delayed gastric emptying is documented in 6–30 % of patients with GERD. Theoretically, gastric stasis can contribute to GERD. However, the relative importance of delayed gastric emptying is not well established [13]. Helicobacter pylori has recently been implicated as having a potential role in the pathogenesis of GERD [14]. H. pylori may secrete proinflammatory substances that can damage esophageal mucosa and sensitize vagal afferent nerves or lead to the reduction of LES tone. In contrast, there are data suggesting a protective role for H. pylori against GERD [15].
Finally, a significant proportion of patients with proven esophagitis do not have increased exposure to acid/pepsin. These patients probably have disruption of mucosal defense mechanisms such as the mucus layer, intercellular junctional complexes, intracellular mechanisms of handling acid, and blood flow to the esophagus [16].
16.2 The Stomach
16.2.1 Anatomic and Physiological Considerations
16.2.1.1 Anatomic Features
The stomach is a storage sac located between the esophagus and duodenum (Fig. 16.1). The proximal stomach consists of the cardia, fundus, and body. The antrum forms the distal stomach and is separated from the duodenum by the pyloric ring. The wall structure of the stomach is similar to that of the rest of the gastrointestinal tract, i.e., it consists of the mucosa, submucosa, muscularis propria, and serosa. However, unlike other parts of the gastrointestinal tract, the muscularis consists of three layers – circular, longitudinal, and oblique. This facilitates distension of the stomach and storage of food. The muscle layer in the antrum is modified to aid the mixing of food. The pyloric ring regulates the emptying of the stomach.
16.2.1.2 Overall Functions
Besides storage, the stomach has a number of exocrine, paracrine, and endocrine functions. The exocrine secretions consist of HCI and pepsin produced by the mucosal parietal cells and chief cells respectively. These cells are located in the fundus and body of the stomach. Most cells within the lamina propria and submucosa are responsible for the main paracrine function, namely, the release of histamine, which in turn stimulates the parietal cells to secrete acid. The antrum secretes the hormone gastrin which enhances gastric emptying and acid secretion.
The intrinsic factor (IF) is a glycoprotein secreted by parietal cells. It binds to Vitamin B-12. The IF-B12 complex in turn binds to specific receptors on the terminal ileal epithelium. Without IF, B12 cannot be absorbed and pernicious anemia develops. Usually failure to secrete IF results from gastric atrophy which causes the destruction of parietal cells.
16.2.1.3 Gastric Motor Physiology
The motor activity of the stomach serves two main functions: (a) to act as a reservoir for ingested meal and ensure timed delivery of food particles to the duodenum at a rate compatible with optimal digestion and (b) to disperse solids into small particles and to mix them with gastric juice. The functions are accomplished by the coordinated activity of three functionally distinct parts of the stomach: (a) the proximal stomach, including the fundus and proximal body; (b) the distal stomach, including distal body and antrum; and (c) the pylorus, as part of the pyloroduodenal unit.
The proximal stomach has three muscle layers – longitudinal, circular, and oblique. No myoelectrical activity is recorded from the fundus during fasting except for the interdigestive migratory motor complexes (see below). In the fed state, the fundus exhibits two forms of motor activity: receptive relaxation and accommodation. Receptive relaxation refers to the reduction in proximal stomach tone initiated by swallowing or pharyngeal stimulation. Accommodation is reflex relaxation of the proximal stomach in response to gastric distention. It is not induced by swallowing or pharyngeal stimulation. Truncal vagotomy abolishes both receptive relaxation and accommodation, suggesting that they are mediated by the vagus nerve. Some gastrointestinal peptides such as cholecystokinin, secretin, VIP, and gastrin induce proximal stomach relaxation, whereas motilin increases fundic pressure [17, 18].
The distal stomach is comprised of two muscle layers: the longitudinal and circular. Slow waves or slow pacer potentials originate in the pacemaker region, located on the greater curvature of the stomach near the junction of the proximal and distal stomach. Slow waves pace the normal 3/min corpus-antral peristalsis, which mixes solid and liquid food with gastric juice and triturates larger particles. The distal gastric motor activity is regulated by cholinergic and noncholinergic, vagal efferent nerve fibers. Cholinergic pathways stimulate antral contraction whereas noncholinergic vagal nerve stimulation inhibits antral activity through VIP and possibly nitrous oxide release from inhibitory neurons within the myenteric plexus.
The pyloric sphincter functions, in coordination with the duodenum, as a sieve allowing particles l mm or smaller to pass into the duodenum in 2- to 4-ml aliquots with each gastric peristalsis [19]. Emptying of inert liquids such as 0.9 % saline follows first-order kinetics; i.e., the volume of liquid emptied into the duodenum in a given time is a constant fraction of the volume that remains in the stomach (Fig. 16.3) [20]. Emptying of digestible solid particles is characterized by a lag phase and a linear phase (Fig. 16.4) [21]. However, the caloric density, viscosity, osmolarity, and volume of any specific meal will influence gastric emptying rates. Fibrous material in the stomach is emptied in the interdigestive state by migrating myoelectrical-contractile complexes comprising 3–10 min of strong lumen-obliterating antral contractions [17].
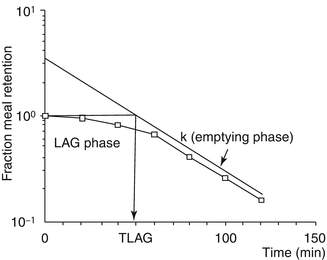

Fig. 16.4
Emptying of digestible solid particles has a lag phase and a linear phase. The curve represents modified power exponential function (From Siegel et al. [21])
16.2.2 Disorders of Gastric Emptying
Conditions that cause abnormal gastric emptying can be divided into two groups: disorders associated with delayed emptying and disorders associated with dumping (Table 16.3). Diabetes is one of the most common causes of delayed gastric emptying in clinical practice. Most afflicted patients have had type I diabetes for more than 10 years, complicated by autonomic and peripheral neuropathy. Delayed emptying of both solids and liquids is attributed to the dysfunction of the proximal and distal stomach, as well as to increased pyloric resistance [22, 23].
Table 16.3
Causes of gastric dysmotility
Condition | Causes |
|---|---|
Mechanical obstruction | |
Gastric outlet obstruction (e.g., tumor, peptic ulcer) | |
Small intestinal obstruction | |
Decreased gastric motility | |
Postsurgical gastroparesis (vagotomy Roux-en-Y, fundoplication, etc.) | |
Endocrine disorders (DM, hypothyroidism, Addison’s disease, hyper- or hypoparathyroidism) | |
Drugs (narcotics, anticholinergics, calcium channel blockers) | |
Connective tissue diseases (e.g., scleroderma, SLE) | |
Muscular disorders (myotonic dystrophy, dermatomyositis) | |
Paraneoplastic | |
Post-viral | |
Neurological disorders (migraine, CVA, Parkinson, dysautonomia) | |
Intestinal pseudo-obstruction | |
Idiopathic gastroparesis | |
Others (anorexia nervosa, uremia, ischemic gastroparesis, pregnancy) | |
Rapid gastric emptying (dumping) | Duodenal ulcer disease (including ZE syndrome) |
Vagotomy | |
Antrectomy | |
Idiopathic |
Idiopathic gastroparesis is also encountered frequently in patients with bloating, early satiety, and nausea. The exact cause is unclear but it may be related to post-viral gastroenteritis [24]. Delayed gastric emptying is a known and frequent complication of gastric surgery. For instance, vagotomy delays emptying of solids but promotes liquid emptying. Similarly, antrectomy can cause rapid emptying of undigested solids and liquids due to the loss of mixing function and loss of pyloric resistance. Symptoms of delayed gastric emptying include early satiety, nausea, vomiting, postprandial abdominal bloating, distention, and pain. The symptoms of gastroparesis are nonspecific and cannot be easily differentiated from those of mechanical obstruction. Other diseases such as gastritis, irritable bowel syndrome, and nonulcer dyspepsia may cause similar complaints.
16.2.3 Duodenogastric Reflux
Duodenogastric reflux (DGR) has been suggested to occur in normal individuals in both fasting and postprandial periods [25, 26], although others have suggested that it does not occur physiologically [27]. The amount of refluxed bile in normal subjects is reported to be small and clears rapidly from the stomach [28]. This feature helps separate normal from abnormal subjects in the fasting state.
Pathologically, DGR has been associated with many conditions (Table 16.4), including postgastrectomy/vagotomy, gastric and duodenal ulcers, cholecystitis, and gastritis [29]. In some of these conditions, such as duodenal ulcer, duodenal hematoma, and cholecystitis, duodenal irritation is probably the underlying mechanism. Other causes may irritate the duodenal mucosa by the adjacent pancreatic inflammation. Slavin also proposed that the deficiency of pancreatic secretions can explain DGR since volume, the alkaline pH, and the physiological components of the pancreatic secretions may be important for maintaining the outward flow of gastric contents [30].
Table 16.4
Causes of duodenogastric reflux
Causes | |
|---|---|
Duodenal ulcer | Gastritis |
Acute cholecystitis | Chronic cholecystitis |
Enteritis | Pancreatitis |
Gastric carcinoma | Surgery |
Post-traumatic stress ulceration | Gastric surgery/vagotomy |
Duodenal hematoma | Cholecystectomy |
Erosive esophagitis | Gallstone dyspepsia |
Physiological/unknown cause |
16.3 The Intestines
16.3.1 The Small Intestine
16.3.1.1 Anatomic and Histologic Consideration
The small intestine is a hollow muscular cylinder that measures 5–6 m in length. It consists of three regions: duodenum, jejunum, and ileum. The intestinal wall is made up of four layers: the mucosa, the submucosa, the muscularis, and the serosa. The small intestinal mucosa is fashioned into villi and crypts to increase the surface area and enhance the absorptive function of the small bowel. The mucosa of the villi consists of absorptive columnar epithelial cells (enterocytes) and mucus-secreting goblet cells. Within the crypts the most common cell type is the undifferentiated crypt cell which secretes chloride and water into the lumen. The crypt also contains pluripotent stem cells. Furthermore, the small bowel harbors the enteroendocrine cells which secrete a number of hormones including secretin, cholecystokinin, gastrin, gastric inhibitory peptide, motilin, glucagon, vasoactive intestinal peptide, somatostatin, and others. These hormones play an important role in gastrointestinal motility. Finally, the intestinal mucosa and lamina propria contain the largest lymphoid organ in man: the gut-associated lymphoid tissue (GALT) [31]. The latter consists of:
M-cells
Intraepithelial lymphocytes (IELs)
Peyer’s patches (lymphoid follicles)
Lamina propria T and B lymphocytes
Dendritic cells
Macrophages
The M-cells are specialized epithelial cells overlying lymphoid follicles. Because they are not covered by mucus, antigens can bind to M-cells which take in the antigens, process them, and present them to lymphocytes and macrophages. However, the binding to antigens is highly selective. For instance, only pathogenic bacteria can attach to M-cells, whereas commensals cannot. IELs are specialized T lymphocytes situated between the basolateral membranes of mucosal epithelial cells and the lamina propria. They appear to have an important immunologic function as they express CD45RO a marker of memory cells. Dendritic cells are derived from the bone marrow and reside beneath M-cells. They capture antigens, carry them across the mucosal barrier, and present them to T lymphocytes.
The submucosa consists of connective tissue, lymphocytes, plasma cells, macrophages, mast cells, fibroblasts, eosinophils, nerve fibers, ganglion cells (Meissner’s plexus), blood vessels, and lymphatics.
The muscularis is made of inner circular and an outer longitudinal muscle fibers. Between these two layers of smooth muscle lies the myenteric plexus, the network of intramural neurons that is essential for all coordinated and organized motor activity. The extrinsic (autonomic) nerves affect the gastrointestinal motility by means of these enteric nerves.
16.3.1.2 Functional Considerations
Most of the digestion and absorption of nutrients takes place in the small bowel. Moreover, the motor function of the small bowel ensures the mixing of chyme with digestive enzymes and the propulsion of chyme toward the colon. Also, the small bowel plays an important role as a first line of defense against pathogenic microorganisms and harmful food antigens.
In most instances, nutrients cannot be absorbed by the cells that line the gastrointestinal tract in the forms in which they are ingested. Digestion is the breakdown of ingested molecules into small ones via reactions catalyzed by enzymes produced by the gastrointestinal organs.
The small intestinal epithelium participates in digestion by secreting oligosaccharidases such as lactase, enterokinase, and peptidases.
Absorption refers to the process of transporting molecules through the epithelial lining of the gastrointestinal tract into the blood or lymph.
Water, electrolytes, monosaccharides, amino acids, small peptides, glycerol, fatty acid, vitamins, and minerals are all absorbed via a number of mechanisms including passive diffusion, facilitated diffusion, active transport, and endocytosis. Although absorption takes place along the entire length of the small intestine, the mucosa in certain regions selectively absorbs specific molecules. For instance, iron is primarily absorbed in the duodenum and proximal jejunum, whereas the terminal ileal mucosa has specific receptors for binding and absorbing vitamin B-12 and bile salts [32].
Under physiological conditions the small bowel exhibits two main motor patterns. During the fed state, and as a result of contact with nutrients, a number of neuronal and hormonal signals are elicited including afferent vagal stimulation and the release of cholecystokinin which mediate segmentation and peristalsis. Segmentation is the most frequent movement in the small bowel and is characterized by closely spaced contractions of the circular muscle layer. These contractions divide the small intestine into short neighboring segments. Segmentation helps mix chyme with digestive enzymes. Peristalsis, on the other hand, is the progressive contraction of successive sections of circular smooth muscle resulting in the propulsion of chyme toward the colon. Furthermore, during the fed state, the small intestine especially the duodenum exerts negative feedback control on gastric emptying via neural and hormonal mechanisms (secretin, cholecystokinin, and gastric inhibitory peptide).
During the fasting phase, the small intestine exhibits a different pattern of motility characterized by bursts of intense electrical and contractile activity separated by periods of lack of activity. This pattern is called migrating myoelectric complex (MMC). In humans MMC occurs every 90–120 min, originates from the stomach, and sweeps through the small bowel till the terminal ileum. The function of MMC is to clear undigested particles and propagate them to the colon [33].
16.3.1.3 Small Intestinal Dysmotility
Motor disorders of the small bowel can lead to symptoms and signs of “functional” as opposed to mechanical small bowel obstruction. Patients frequently complain of abdominal distension, bloating, and abdominal pain, and when small intestinal dysmotility is associated with gastroparesis, nausea and vomiting may be prominent. On physical examination, the abdomen is usually distended and bowel sounds are diminished. Features of bacterial overgrowth may be observed.
Small intestinal dysmotility may be acute or chronic. Acute dysmotility is termed adynamic ileus and is commonly seen following abdominal surgery, severe septicemia, or electrolyte disturbances such as hypokalemia. Chronic dysmotility is termed pseudo-obstruction (Table 16.5).
Table 16.5
Motor disorders of the small intestine
Cause | Mechanism | Outcome |
|---|---|---|
Acute illness | Impaired smooth muscle contraction | Adynamic ileus |
Altered neurotransmission | ||
Pregnancy | Decreased smooth muscle contraction (progesterone) | Slow transit |
Diabetes mellitus | Autonomic dysfunction | Slow or rapid transit |
Scleroderma | Smooth muscle fibrosis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|