Cartilage injuries in the knee are common and can be a persistent source of pain or dysfunction. Many new surgical strategies have been developed to treat these lesions. It is important for the radiologist to have an understanding of these procedures and their appearance on magnetic resonance (MR) imaging. This article provides the radiologist with an overview of the surgical strategies for repairing cartilage lesions in the knee followed by a discussion of their postoperative appearance on MR imaging in normal and abnormal cases. Guidelines for adequate reporting of the MR imaging findings after cartilage repair in the knee are also included.
Key points
- •
Current cartilage repair strategies in the knee.
- •
Magnetic resonance (MR) imaging appearance of cartilage repair procedures in the knee.
- •
Reporting MR imaging findings of cartilage repair in the knee.
Introduction
Articular cartilage lesions occur frequently and can be a common source of pain, especially in the knee. Up to 36% of athletic injuries have an associated chondral injury, more so in women. A study of 31,516 arthroscopies identified chondral injuries in 63% of patients. When left untreated, chondral lesions can accelerate the development of osteoarthritis and result in permanent dysfunction. Mature articular cartilage has limited capability for repair because of poor vascularity, inability to recruit undifferentiated cells, and limitations on cell replication and migration by the dense extracellular matrix. Some investigators have suggested that a subset of cartilage defects in humans may heal spontaneously, and spontaneous cartilage repair has been shown in some animal models. However, even in animal models, the repair tissue is imperfect and dependent on the age of the animal. For these reasons, there has been intense research in the development of techniques to repair or restore articular cartilage surfaces.
Imaging plays an increasingly important role in both the initial detection of chondral lesions and the postoperative evaluation of chondral repair procedures. Magnetic resonance (MR) imaging, with its ability to directly image cartilage and chondral repair tissue, is particularly valuable and with proper use may help patients avoid unnecessary arthroscopic or even open surgery. Because an increasing number of cartilage repair procedures are being performed, the radiologist must have an understanding of the surgical techniques involved, the expected postoperative appearances of these repairs, and the possible complications associated with repair strategies. The basic approaches to the treatment of cartilage damage include nonoperative conservative measures, lavage and debridement, marrow stimulation techniques, synthetic scaffolds, biological tissue grafting, and cell-based therapies. Many cartilage repair techniques are not approved by the US Food and Drug Administration and are available only outside the United States or through clinical trials.
The following sections provide an overview of current cartilage repair strategies and their normal and abnormal MR imaging appearances. Guidelines for reporting postoperative imaging findings are discussed also.
Introduction
Articular cartilage lesions occur frequently and can be a common source of pain, especially in the knee. Up to 36% of athletic injuries have an associated chondral injury, more so in women. A study of 31,516 arthroscopies identified chondral injuries in 63% of patients. When left untreated, chondral lesions can accelerate the development of osteoarthritis and result in permanent dysfunction. Mature articular cartilage has limited capability for repair because of poor vascularity, inability to recruit undifferentiated cells, and limitations on cell replication and migration by the dense extracellular matrix. Some investigators have suggested that a subset of cartilage defects in humans may heal spontaneously, and spontaneous cartilage repair has been shown in some animal models. However, even in animal models, the repair tissue is imperfect and dependent on the age of the animal. For these reasons, there has been intense research in the development of techniques to repair or restore articular cartilage surfaces.
Imaging plays an increasingly important role in both the initial detection of chondral lesions and the postoperative evaluation of chondral repair procedures. Magnetic resonance (MR) imaging, with its ability to directly image cartilage and chondral repair tissue, is particularly valuable and with proper use may help patients avoid unnecessary arthroscopic or even open surgery. Because an increasing number of cartilage repair procedures are being performed, the radiologist must have an understanding of the surgical techniques involved, the expected postoperative appearances of these repairs, and the possible complications associated with repair strategies. The basic approaches to the treatment of cartilage damage include nonoperative conservative measures, lavage and debridement, marrow stimulation techniques, synthetic scaffolds, biological tissue grafting, and cell-based therapies. Many cartilage repair techniques are not approved by the US Food and Drug Administration and are available only outside the United States or through clinical trials.
The following sections provide an overview of current cartilage repair strategies and their normal and abnormal MR imaging appearances. Guidelines for reporting postoperative imaging findings are discussed also.
Nonoperative conservative therapy, lavage, and debridement
Nonperative conservative treatments include symptomatic relief options such as nonsteroidal antiinflammatory drugs, braces, oral nutriceuticals such as chondroitin sulfate, intra-articular viscosupplementation with hyaluronic acid (HA), and injection with platelet-rich plasma (PRP). No studies have shown a significant improvement in the MR imaging appearance of chondral surfaces after HA or PRP injection. This finding could, in part, relate to the short follow-up interval of these studies (1–2 years). In 1 randomized controlled study, clinical outcome scores were better for patients who underwent PRP injection than for those who received HA injection.
Lavage and debridement are usually performed arthroscopically and involve the removal of unstable cartilage, loose bodies, and osteophytes. After debridement, lesions may appear larger and may have sharper margins on MR imaging. Some lesions may appear to fill, either from attempted self-repair or because of differences in slice position and partial volume averaging artifacts. MR imaging is usually performed after these therapies to assess the joint for progression of the cartilage defect or development of new structural damage.
Surgical techniques for cartilage repair
All of the surgical techniques begin with complete removal of the damaged cartilage. This procedure may involve debridement of the cartilage to the calcified cartilage or subchondral bone. Bone and cartilage may also be removed en bloc or as a plug (as in osteochondral grafting). As a result, all tissue within a cartilage repair site represents either newly grown or implanted tissue. Tissue grafting techniques use autograft or allograft tissue to provide a more optimal hyaline or hyalinelike cartilage repair tissue. Cell-based therapies are 2-step procedures in which chondrocytes harvested from the patient are cultured and then reimplanted as cells or as cell-seeded scaffolds. Acellular scaffolds may also be implanted into prepared cartilage defects. Nonbiological therapies can provide partial or complete resurfacing of a single compartment of a joint with metal or polyethylene, but these treatments are beyond the scope of this article. Patient compliance with a proper rehabilitation regimen is necessary to ensure a good outcome. A basic algorithm for these various procedures is shown in Fig. 1 .
Bone Marrow Stimulation
Marrow stimulation techniques are probably the most common surgeries for chondral defects, with microfracture being the most common of these. The goal of stimulation procedures is to promote bleeding that forms a clot, which then fills the chondral defect with pluripotent cells derived from bone marrow. Cytokines are released, stimulating the formation of fibrocartilage within the lesion. However, fibrocartilage does not have the same biomechanical properties as native hyaline cartilage, so this repair may be less durable.
Early marrow stimulation procedures removed the subchondral bone plate altogether. These procedures were then replaced by abrasion arthroplasty, a less invasive subchondral bone plate debriding technique. As time progressed, less invasive procedures were favored, such as subchondral bone drilling and microfracture. Although drilling and microfracture are similar, historically, drilling was theoretically believed to have a higher risk of tissue necrosis. However, a recent study in a rabbit model showed more osteocyte necrosis with microfracture than with microdrilling.
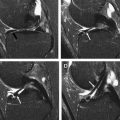
Microfracture is appealing because it can be performed arthroscopically, is therefore relatively noninvasive, and has low morbidity. In this procedure, the chondral lesion is first debrided to sharp, stable margins, and an awl is then used to create 1-mm to 2-mm holes in the subchondral bone plate approximately 3 to 5 mm apart across the entire lesion area ( Fig. 2 , [CR] ). The recovery and rehabilitation time for microfracture is the same as for cartilage repair surgeries for small lesions (<4 cm 2 ): 6 weeks on crutches with partial weight bearing and continuous passive motion for 6 to 8 hours daily. No inline running for 6 to 9 months and no pivoting sports for 1 year are advised.
Microfracture has the best outcomes when the patient age is younger than 40 years, the procedure is performed within 12 months of the initial injury, and the lesions are less than 2 cm 2 . Outcomes are not as good for microfracture performed in the patellofemoral compartment and for lesions greater than 4 cm 2 . The long-term durability of cartilage repair by microfracture seems to be limited; integrity has been shown to decline at approximately 18 to 36 months. The formation of intralesional osteophytes has been described as a mode of failure. Although failed microfracture repair sites can be revised with other cartilage repair techniques, there is evidence that postoperative changes in the subchondral bone plate may reduce the success rate of the second procedure.
Recently, techniques have been proposed to improve the microfracture technique by adding a scaffold or polymer to the repair site. The purpose of the collagen matrix is to promote the formation of a higher-quality repair tissue. For an Autologous Matrix-Induced Chondrogenesis (AMIC) procedure, a collagen I/III porcine matrix is fixed within the defect with fibrin glue. Alternatively, a chitosan-glycerol phosphate polymer (Piramal Healthcare, Laval, Quebec, Canada) (marketed as BST-CarGel in Europe) may be added to the microfracture site to help stabilize the blood clot and reduce contraction of the clot. Additional methods of augmenting microfracture using biochemical modifiers such as growth factors and gene therapy have been proposed.
Tissue grafts
Tissue grafting replaces the damaged articular surface with tissue from the same patient (ie, orthotopic autograft) or from a deceased donor (ie, allograft). With osteochondral grafts, bone and its overlying cartilage are transplanted, making the repair a true hyaline articular cartilage. More recently, particulated articular cartilage has been used for repair as an autograft (CAIS) or allograft juvenile cartilage (DeNovo NT). Processed allograft tissue has also recently been used as an acellular biological scaffold to promote growth of bone and cartilage (eg, ChondroFix and ACS Articular Cartilage Scaffold).
Osteochondral Allograft
Osteochondral allograft uses cadaveric donor tissue to repair a bone-cartilage defect. This technique is most often used as a second-line salvage procedure for larger lesions, typically greater than 2.5 cm 2 , which may have bone loss, but is also used for primary treatment of large defects in active patients with high physiologic demands. Chondrocyte viability in allograft material is dependent on tissue handling. The donor tissue must be harvested within 24 hours of death. Allografts may be fresh (stored at 4°C in culture) or frozen (stored at −40°C). Because freezing can kill chondrocytes, many surgeons prefer fresh over cryopreserved specimens. The allograft bone is avascular and nonviable but provides a rigid structure to support the chondral allograft until it is incorporated by native bone. Chondrocytes are believed to be immunoprivileged; however, there have been reports of immunoreactivity of chondrocytes after osteochondral allograft procedures. Because the marrow elements are removed and the amount of transplanted bone is minimized, the risk of immunologic complications is decreased. In a review of the literature, Rihn and Harner found no reports of clinically significant immune reactions after musculoskeletal allograft transplantation. For these reasons, donor tissue matching and immunosuppression therapy are not required for osteochondral allograft procedures. The risk of disease transmission is low, reported to be equivalent to that of a blood transfusion. Buck and colleagues reported that if the donors are properly screened for infection, the estimated risk of human immunodeficiency virus transmission is 1 in 1.6 million.
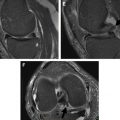
In the osteochondral allograft procedure ( Fig. 3 ), the lesion is first debrided to healthy bone. Perforations are then created in the native bone to promote bleeding from the marrow space, which aids in native bone ingrowth. An allograft bone-cartilage segment is either tailored to the geometry of the lesion or consists of a large single round plug. If the allograft segment cannot be adequately press fit into the donor site, a screw or pin, often made of a bioabsorbable material, may be used. Overall, outcomes for this procedure are good, with high allograft survival rates: 85% at 4 years in 1 study and 72% at 7.7 years in another study for lesions averaging 8 cm 2 . For very large lesions greater than 10 cm 2 , a unicompartmental prosthesis may be more appropriate.
Osteochondral Autograft
Osteochondral autograft procedures make use of the patient’s own bone-cartilage surfaces for donor material. Other commonly used terms for this procedure include generic names such as autogenous osteochondral transfer (AOT) and proprietary names such as osteochondral autograft transfer system (OATS) and mosaicplasty, which are frequently used as generic names. These procedures allow the defect to be resurfaced with native hyaline cartilage with no risk of disease transfer and no need to wait for donor tissue. Osteochondral autograft is typically used for lesions greater than 1 cm 2 but less than 4 to 5 cm 2 and up to 10 mm deep.
The donor osteochondral material is typically harvested from the symptomatic joint from a relatively non–weight-bearing area, most often the lateral trochlea near the sulcus terminalis, but the margins of the intercondylar notch and medial trochlea are also options. Donor material may also be obtained from other joints (eg, treatment of a lesion of the talus or elbow with a donor site in the knee). When the lesion is small, it may be completely filled with 1 cylindrical osteochondral plug ( Fig. 4 ). Larger lesions are typically filled with several plugs to better recreate the articular contour. Donor sites greater than 6 mm in diameter have higher rates of donor site morbidity. Plugs of approximately 4.5 mm in diameter have a lower risk of breakage during implantation and a lower rate of donor site morbidity than larger plugs. The term mosaicplasty has been coined for the use of multiple plugs because the repair tissue resembles a mosaic, with a mixture of hyaline cartilage tiles and fibrocartilage grout filling the space between the transferred osteochondral plugs. Typically, the donor sites are left as empty osteochondral defects, which fill over time with bone covered by fibrocartilage. Imaging evaluation of these osteochondral autografts should include both the donor and recipient sites.
Particulated Cartilage Tissue Graft
Chondrocytes within articular cartilage are embedded within a dense extracellular matrix and have a limited ability to migrate. However, when cartilage is cut into small particles, the cells are liberated from the matrix and proliferate. A particulated or minced adult chondral autograft was first shown to effect a chondral repair in a rabbit model in Germany. The combined change in local environment from the mincing process and fibrin glue and possibly the exposure to subchondral bleeding are believed to modify adult chondrocytes to behave more like juvenile chondrocytes, thus producing extracellular matrix and realizing a hyalinelike repair. Unlike the hyaline cartilage that is transferred with osteochondral grafts, the cartilage repair tissue that grows from particulated cartilage becomes incorporated into the surrounding articular cartilage.
As an autograft procedure performed through a miniarthrotomy, CAIS is a 1-step procedure in which roughly 200 mg of the patient’s own cartilage is harvested from the margin of the intercondylar notch as minced cartilage particles. These particles are deposited uniformly onto a biodegradable scaffold and covered with fibrin glue. The coated scaffold is then trimmed to fit the debrided lesion and affixed to the defect with bioabsorbable staples, with the particulated cartilage facing the subchondral bone. CAIS is limited by the amount of donor cartilage that may be harvested and the potential of donor site morbidity. This procedure is available in Europe but has not been approved in the United States.
An allograft approach to particulated cartilage transplantation, DeNovo NT, has been developed using juvenile donor cartilage. DeNovo NT is harvested from donors ranging in age from 29 days to 12 years (mean age, 4.2 years). The method exploits the potential advantage of providing cartilage particles with a greater cell density, which may produce more extracellular matrix. In addition, a greater amount of cartilage may be grafted, because there is no potential for donor site morbidity, as there is with autograft techniques. As mentioned earlier, cartilage is immunoprivileged; no immunologic reactions to chondral allograft have been reported. This material is considered to be minimally manipulated tissue and is approved for use in the United States. During the single-stage procedure, the lesion is debrided, with removal of the calcified layer of cartilage and with efforts made to keep the subchondral bone plate intact. Two methods for filling the debrided chondral defect have been suggested. One option is to create a mold of the debrided lesion with foil, fill the mold with evenly distributed tissue particles and fibrin glue, and allow the mixture to set for 3 to 10 minutes. This cartilage–fibrin glue construct is then transferred and fixed within the chondral defect with fibrin glue. The alternative method is to fill the debrided chondral defect directly with evenly distributed particulated juvenile chondrocytes and fibrin glue. The DeNovo NT graft is intentionally underfilled by at least 1 mm, and usually 2 to 3 mm, to protect the new repair tissue in the immediate postoperative state. DeNovo NT has been successfully used to fill bone defects less than 6 mm deep.
Cell and Tissue Culture Therapies
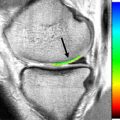
Autologous chondrocyte implantation (ACI) was developed in the 1980s and spurred much of the interest in cell-based cartilage repair techniques. Since the creation of the first generation of techniques, which used periosteum to cover the defect, several variations of ACI have been developed. ACI is a 2-step procedure. First, 250 to 300 mg of cartilage is harvested arthroscopically from a less weight-bearing portion of the joint; in the knee, this is typically the margin of the intercondylar notch or trochlea. The chondrocytes are extracted from the harvested cartilage and cultured ex vivo under special conditions for 3 to 4 weeks to expand the number of cells. During a second surgery (usually open), the lesion is debrided, a periosteal (first-generation, ACI-P) or synthetic collagen cover (eg, collagen-covered autologous chondrocyte implantation [second-generation CACI or ACI-C]) is sewn over the defect to the adjacent articular cartilage, and the cultured chondrocytes are implanted beneath the cover ( Fig. 5 , [CR] ). With this technique, large defects may be repaired, because the amount of harvested material does not significantly limit the number of cultured cells that may be provided. Although some have reserved ACI as a second-line therapy (ie, after failure of marrow stimulation procedures), increased failure rates have been seen with ACI when used as a second-line procedure. Larger cartilage defects may be best treated with primary ACI. The best results have been seen in younger patients and in those with single medial femoral condyle lesions ; however, good results have also been seen in patients with multiple lesions, with greater than 92% of patients with osteoarthritis functioning well for greater than 5 years, delaying joint replacements. The hyalinelike repair tissue seems to be durable, as shown by 2 case series of greater than 200 patients each; in these studies, 74% to 75% of patients had improved function greater than 10 years after surgery.
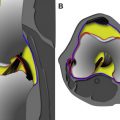
More recently, scaffolds have been incorporated into ACI techniques to support the implanted chondrocytes and optimize repair. The chondrocytes extracted from the harvested cartilage are grown within a collagen matrix ex vivo and then inserted as a cultured tissue ( Fig. 6 ). This third-generation technique is often referred to as matrix-assisted autologous chondrocyte implantation, although matrix-assisted chondrocyte implantation (MACI) is also a proprietary name for a specific technique. The scaffold may be made of one of several materials including collagen (MACI, NeoCart) or HA (Hyalograft C); each product has proprietary culture techniques. The scaffold is stabilized in the repair site with fibrin glue or collagen-based glue and usually does not completely fill the depth of the defect. Therefore, no suturing is required and no periosteal or collagen patch is needed. Furthermore, these procedures may be performed with smaller arthrotomies or arthroscopically.
With all of the chondrocyte implantation techniques, every effort is made to preserve the underlying subchondral bone to minimize cell contamination by subchondral marrow, which may induce a fibrocartilage repair tissue. Posttreatment evaluation of cell-based tissue implantation therapies shows the repair tissue to be hyalinelike with a mix of fibrocartilage compared with the more homogeneously fibrocartilage repair of marrow stimulation techniques. Graft hypertrophy (overgrowth of the repair tissue above the level of the adjacent articular surface) was a common but treatable complication with first-generation ACI techniques, which was believed to be caused by the periosteal cover. The incidence of this complication has been greatly reduced with the use of a collagen membrane cover and with cultured scaffold techniques.
An allograft-seeded scaffold, RevaFlex (formerly DeNovo ET), currently in phase 3 clinical trials, combines strategies by culturing particulated allograft juvenile cartilage on a scaffold ex vivo for implantation in a 1-step procedure. This engineered tissue is available in 22-mm to 24-mm disks, which can be tailored to fit the lesion at the time of implantation and are affixed to the lesion with fibrin glue.
Implanted Scaffold Therapies
In another strategy for cartilage repair, synthetic scaffolds engineered to promote cartilage or bone growth are implanted within the cartilage defect. Several different materials have been proposed for this use. These scaffolds may be cell seeded, loaded with growth factors or blood products, or acellular. The acellular scaffolds promise a 1-step off-the-shelf solution to cartilage repair. Most of these technologies are in development or in early trials. An acellular scaffold that is commercially available in Europe (TruFit CB or BGS) has shown conflicting short-term to intermediate-term results. These cylindrical scaffolds are composed of 2 polymers: one designed to promote chondrocyte growth at the surface and the other designed to promote bone growth at the base. One study reported improved clinical scores, development of a cartilagelike surface based on delayed gadopentetate-enhanced MR imaging of cartilage (dGEMRIC) MR imaging features, and no damage to opposing articular surfaces. However, another study comparing TruFit BGS scaffolds with mosaicplasty showed better clinical results at a mean of 22 to 30 months follow-up for mosaicplasty. By MR criteria, good incorporation of the bone and cartilage repair to the surrounding tissues has been reported in patients but was shown to be ongoing at 16 months ( Fig. 7 ). Although patients may return to weight-bearing activities relatively quickly after these procedures are performed, the imaging appearance of these repair sites may appear concerning for years while the tissue ingrowth matures. A study by Barber and Dockery indicates that bony incorporation or ingrowth into this scaffold is either extremely slow or incomplete, with persistent areas of the scaffold without bone, as shown by computed tomography (CT) scans, even after 5 years. A trilayer nanostructured biomimetic scaffold (MaioRegen, Fin-Ceramica Faenza SpA, Faenza, Italy) has shown promising results in the experimental stages. The 2-year and 5-year follow-up studies have shown good clinical outcomes with statistically significant clinical improvements when compared with preoperative and 2-year time points, respectively. The magnetic resonance observation of cartilage repair tissue (MOCART) scores, although shown to significantly improve with time, were not as favorable as clinical findings, and no correlation was noted between clinical outcomes and MR imaging findings even after 5 years. As mentioned in the section on allografts, treated acellular allogeneic cartilage-bone cylinders that serve as scaffolds are also available for implantation.
MR imaging assessment of surgical cartilage repair
Arthroscopy is considered the gold standard for postoperative assessment of surgical cartilage repair; however, this procedure is invasive and relatively expensive. Arthroscopic evaluation may include the degree of filling of the defect, the integration of the edge (border zone) of the repair tissue to the surrounding articular cartilage, the macroscopic appearance of the repair tissue (smooth, fraying/fissuring), the color of the tissue, and the stiffness of the tissue on probing. The International Cartilage Repair Society macroscopic evaluation of cartilage repair and Oswestry Arthroscopy Score are semiquantitative scoring systems that provide an overall grade of the success of the cartilage repair and are useful for research and clinical trials.
Although MR imaging cannot provide an accurate assessment of some of the arthroscopic features of cartilage repair tissue such as color, stiffness, and fine fibrillations, it can provide information about the defect fill and integration along with additional information about the subchondral bone. Because this technique is noninvasive and easily repeated, imaging is generally a more practical way to assess surgical success than arthroscopy. MR imaging is now used frequently in clinical trials of new cartilage repair techniques. Although follow-up MR imaging at regular intervals is commonly performed in clinical trials, in clinical practice, MR imaging is reserved for symptomatic patients and is often the one of the first steps, because it provides valuable information about the repair site structure. The information provided can help clinicians to plan surgical or nonsurgical interventions. Blackman and colleagues reviewed 26 studies assessing the correlation between MR imaging and clinical outcomes, concluding that imaging findings at 6 and 36 months postoperatively correlated best with clinical outcomes. In the very early postoperative phase, many repairs are immature, and imaging before 6 months after surgery may confuse the clinical picture, unless warranted by acute symptoms or to assess for non–repair site complications. Often, repair site findings found on MR do not correlate with symptoms, but the structural abnormalities identified by imaging may have prognostic implications. Some surgeons may request MR imaging for asymptomatic patients when the patient is considering a change in activity level. MR findings of an immature repair may factor into a decision to delay their return to high-demand activities.
Several MR scoring systems have been developed to help standardize postoperative cartilage assessment. Most of these systems judge many parameters, and although these systems are useful in gathering objective data for clinical trials, they may not be practical for daily radiology reports. However, a review of these systems can help provide the basis for an effective clinical radiology report.
In 2003, Henderson and colleagues reported on the use of an MR scoring system to assess cartilage repair tissue after ACI. The Henderson scoring system assigns a score of 1 to 4 points in 4 categories, with lower scores indicating a better result, the opposite of arthroscopic scoring systems. The Henderson MR imaging parameters are defect filling (complete fill = 1 point; >50% = 2; <50% = 3; full-thickness defect = 4), repair tissue signal intensity (normal = 1; near normal = 2; abnormal = 3; absent signal = 4), bone marrow edema (absent = 1; mild edema = 2; moderate edema = 3; severe edema = 4), and joint effusion (absent = 1; mild = 2; moderate = 3; severe = 4). In this study, significant improvements in appearance were seen postoperatively at 12 months compared with 3 months. For 15 patients who also had a postoperative arthroscopy, moderate correlation between the Henderson score and the arthroscopic visual data was reported. No single parameter or total score correlated well with the clinical outcomes. Another MR imaging scoring system reported by Roberts and colleagues in the same year also used 4 categories, assigning either 0 (abnormal) or 1 (normal or nearly normal) point for each category, with a score of 4 points indicating the best possible result. The 4 MR parameters in this system are surface integrity and contour, signal intensity of the repair tissue, repair cartilage thickness, and changes in the underlying bone. The study reported a correlation between MR scoring and histologic scoring of postoperative biopsy specimens. Although this system is simpler, it does not provide many gradations for abnormalities.
In 2004, Marlovits and colleagues defined the MR parameters of the MOCART scoring system, which showed sensitivity to changes between 6 and 12 months after surgery for 45 patients who had undergone surgical cartilage repair with microfracture, MACI, or autologous osteochondral transplantation. Nine parameters were defined to assess the quality of the repair: defect filling, repair tissue integration, repair tissue surface, repair tissue structure, repair tissue signal intensity, subchondral lamina status, subchondral bone status, presence or absence of adhesions, and presence or absence of synovitis. In 2009, Welsch and colleagues proposed 3D-MOCART as a modified version of the MOCART system adapted for higher-resolution imaging, including isotropic three-dimensional acquisitions. This version refined gradations for some parameters, included localization and degree of involvement relative to the repair site, divided repair tissue integration into cartilage and bone interfaces, added parameters for chondral osteophytes and bone marrow edema, eliminated adhesions, and replaced synovitis with effusion. The MOCART systems are the most commonly used MR scoring systems for clinical trials of cartilage repair.
A recent publication suggests that 1 scoring system may not be optimal for all cartilage repair procedures. The investigators indicate that the MOCART system, although comprehensive for marrow stimulation and cell-based therapies, may not include adequate assessment of the bony changes to fully evaluate osteochondral allografts. The Osteochondral Allograft MRI Scoring System (OCAMRISS), on the other hand, grades 5 cartilage parameters, 4 bone parameters, and 4 additional joint findings and was compared with micro-CT and histopathologic evaluations in a goat model with substantial agreement for almost all parameters.
The number of MR scoring systems for cartilage repair and the detailed parameters discussed earlier can present a challenge for a high-volume clinical radiologist. Distilling the necessary features to generate a radiology report that is useful to the cartilage repair surgeon is important for optimal patient care. Table 1 summarizes the important features that we recommend be mentioned in the report, and the following sections describe the details of these features.