Male Genital Tract
Andrew Phelps
Jesse Courtier
Peter “Buzz” Marcovici
Sara O. Vargas
John D. MacKenzie
INTRODUCTION
Abnormalities of the genital tract in infants and children can be either congenital or acquired and commonly require imaging assessment. In particular, scrotal pain and intrascrotal palpable abnormalities are common indications for imaging in both the emergency department and outpatient settings. This chapter reviews imaging techniques used to evaluate the male genital tract as well as discusses the clinical and radiologic features of various conditions affecting the pediatric scrotum, testicles, epididymides, seminal vesicles, and prostate gland.
IMAGING TECHNIQUES
Radiography
Radiography of the male genital tract is of limited value in the pediatric population. However, in addition to an inguinal hernia containing the intestine, a pediatric genital tract mass such as testicular and paratesticular neoplasm presenting as an enlarged soft tissue mass or other lesions associated with calcification may be incidentally detected on radiographs. Such radiographic information can guide decision making for subsequent imaging study for further evaluation.
Ultrasound
Imaging of the scrotum, like genitourinary imaging in general, relies principally upon ultrasound, with other imaging modalities playing secondary roles. A high-frequency (9 to 18 MHz) linear transducer is ideal for obtaining high-resolution grayscale images of the testicles, epididymides, and inguinal canals. Testicular volume can be estimated by ultrasound by measuring the longitudinal, transverse, and anteroposterior diameters of the testicle and multiplying by a constant (either 0.52 or 0.71).1 Testicular volume measurements are useful for identifying subtle size asymmetry and comparing with standardized growth charts.2,3 Cine sweeps through the testicles help exclude the presence of small testicular lesions that may be missed by representative still ultrasound images.
It is also important to demonstrate symmetric and uniform blood flow in the testicles and surrounding soft tissues (including epididymides) with Doppler ultrasound (color, power, and spectral Doppler); a side-by-side color or power Doppler image of both testicles in the transverse plane is helpful for detailing symmetric blood flow and is preferred by some as the first image obtained in the workup of acute scrotal pain. Dedicated gray-scale and Doppler ultrasound images also should be obtained of the epididymides and inguinal canals. At some institutions, ultrasound images of the urinary bladder and kidneys are also obtained as part of a routine scrotal ultrasound, with the thought that acute renal abnormalities can mimic acute scrotal pathology (e.g., pain from obstructing urolithiasis can cause inguinal or scrotal pain).
A transabdominal approach with ultrasound, when the bladder is adequately distended with urine, is most often used to image the seminal vesicles and prostate gland in children. These structures can also be further evaluated with computed tomography (CT) or magnetic resonance imaging (MRI) when ultrasound is inadequate. In older children, transrectal ultrasound imaging of the prostate gland and seminal vesicles can also be performed.
Computed Tomography
If a testicular mass is identified, cross-sectional imaging of the abdomen and pelvis with contrast-enhanced CT may be
employed to facilitate tumor staging, with particular attention paid to retroperitoneal lymph nodes. In general, CT does not aid in the evaluation of the testicular mass itself, except in situations where the mass is larger than the footprint of an ultrasound transducer and local extension may be better defined with the larger field of view afforded by CT. Infrequently, CT may be used to evaluate the prostate gland or seminal vesicles (e.g., in the setting of a known or suspected mass).
employed to facilitate tumor staging, with particular attention paid to retroperitoneal lymph nodes. In general, CT does not aid in the evaluation of the testicular mass itself, except in situations where the mass is larger than the footprint of an ultrasound transducer and local extension may be better defined with the larger field of view afforded by CT. Infrequently, CT may be used to evaluate the prostate gland or seminal vesicles (e.g., in the setting of a known or suspected mass).
Magnetic Resonance Imaging
To eliminate ionizing radiation exposure associated with CT, MRI can also be employed for testicular tumor staging or definition of locoregional tumor extension. In addition, MRI is helpful for characterizing disorders of sex development (DSD) when ultrasound fails to adequately depict or characterize the gonads or uterus. Diffusion-weighted MR imaging, in particular, provides a sensitive means of detecting gonads, which can be otherwise difficult to detect by ultrasound or even CT when they are intra-abdominal in location. MRI also may be used to evaluate the prostate gland or seminal vesicles (e.g., in the setting of a known or suspected mass or cystic abnormality).
Nuclear Medicine
Historically, nuclear medicine evaluation of the scrotum using 99mTc-pertechnetate scintigraphy was performed to assess for altered testicular perfusion in the setting of suspected torsion; currently, ultrasound has replaced this technique. 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) imaging may be used in the initial staging and follow-up of certain male genital tract malignancies.
NORMAL ANATOMY
Embryology
The male genital tract is composed of the scrotum, testicles, epididymides, vas deferentia, seminal vesicles, prostate gland, and penis. In the sexually undifferentiated embryo, the paired gonads are located high in the retroperitoneum. The sex-determining region on the short arm of the Y chromosome (SRY gene) causes germ cells to differentiate into Sertoli cells (which produce Mullerian-inhibiting factor) and Leydig cells (which produce testosterone); together this leads to gonadal transformation into testicles, descent into the scrotal swellings, and near-complete regression of the paramesonephric ducts (discussed more below).4 Testicular descent is guided by the gubernaculum (also called the caudal genital ligament) in conjunction with an inguinal outpouching of the peritoneal cavity, the processus vaginalis, which ultimately mostly obliterates and no longer communicates with the peritoneal cavity.
Once the testicle has descended into the scrotum, the scrotal portion of the processus vaginalis (also known as the tunica vaginalis) should cover the majority of the testicle, though leaving a broad “uncovered” portion of the testicle which is in direct contact with the internal spermatic fascia (the fascia is an evagination of the transverse abdominal wall fascia).4 The layer of the tunica vaginalis that contacts the testicle is called the lamina visceralis and the remainder is called the lamina parietalis. All but the caudal most portion of the gubernaculum involutes in males, leaving what is called the gubernaculum testis (also called the scrotal ligament), which serves to anchor the caudal aspect of the testicle to the scrotum.
The epididymides, ejaculatory ducts, and seminal vesicles arise from the paired mesonephric ducts (also known as Wolffian ducts). This is covered in more detail in the kidney chapter (Chapter 17) in this book. The prostate gland arises from a combination of the mesonephric ducts and urogenital sinus. The paramesonephric ducts (also known as Mullerian ducts) give rise to the upper portion of the vagina, uterus, and fallopian tubes in females, but nearly completely regress in males; the only remnant is a tiny midline posterior outpouching of the prostatic urethra (prostatic utricle) located at the verumontanum.5 In females, the mesonephric ducts nearly completely regress; the paravaginal Gartner ducts (and sometimes seen Gartner duct cysts) are persistent remnants. These gender-specific changes are illustrated in Figure 18.1.
The penis develops from several tissues surrounding the opening of the urogenital sinus.6 The genital tubercle arises dorsal (superior) to the urogenital sinus opening and becomes the erectile tissue: corpora cavernosa and glans penis (versus clitoris in female). The mesenchyme surrounding the penile urethra gives rise to the corpus spongiosum. The urogenital folds are located to the sides of the urogenital sinus opening and become the foreskin (versus labia minora in females). The labioscrotal folds lie on either side of the urogenital folds and form the external scrotal layer (versus labia majora in females).
Normal Anatomy
The scrotal sac (lined by tunica vaginalis) may contain trace simple fluid in normal children. When the scrotal sac is pathologically fluid filled (e.g., hydrocele), the fluid surrounds the covered portion of the testicle. There should normally be no communication between the scrotal sac and the peritoneal cavity, as the inguinal portion of the processus vaginalis closes. Also, a midline septum divides the scrotum into two portions, further limiting movement of intrascrotal structures and fluid.
The testicles normally have a characteristic ovoid shape and homogeneous echotexture on gray-scale ultrasound (Fig. 18.2). The testicular surface is normally smooth, correlating with the tunica albuginea (a connective tissue layer covering testicular parenchyma and that itself is covered by the tunica vaginalis). Arterial and venous spectral Doppler waveforms should be readily detectable in most children (Fig. 18.3), although Doppler signal can be challenging to detect in the normal testicles of very young boys. Normal testicular volume in the newborn male averages 0.3 mL and increases to 0.5 mL in the first year of life, after which the
volume increases minimally until puberty, when the volumes rapidly increase to the adult volume of 13 to 17 mL.2,3 As the testicle enlarges with puberty, the more hyperechoic testicular mediastinum (a connective tissue structure that contains the rete testis and is the interface between epididymis and testicle) becomes more apparent extending from the superior to inferior portion of the testicle posteriorly. The mediastinum testis gives off fibrous trabeculae that course toward the tunica albuginea dividing the testicle into lobules.
volume increases minimally until puberty, when the volumes rapidly increase to the adult volume of 13 to 17 mL.2,3 As the testicle enlarges with puberty, the more hyperechoic testicular mediastinum (a connective tissue structure that contains the rete testis and is the interface between epididymis and testicle) becomes more apparent extending from the superior to inferior portion of the testicle posteriorly. The mediastinum testis gives off fibrous trabeculae that course toward the tunica albuginea dividing the testicle into lobules.
 FIGURE 18.1 Embryology and sexual differentiation of the genitourinary system is schematized. Color-coded components include urogenital sinus (yellow), gastrointestinal tract (green), mesonephros/Wolffian ducts (blue), metanephros (red), paramesonephros/Mullerian ducts (pink), gonads (brown), genital tubercle (orange), and labioscrotal folds (purple). (Reproduced with permission from RSNA and the copyright owner.120) |
The head of the epididymis is located adjacent to the superior pole of the testicle and is normally smaller than the testicle. The epididymal head is approximately semiovoid, often with a visible small cleft separating it from the testicle (Fig. 18.2). The body and tail of the epididymis are small tubular structures hugging the long axis of the testicle and contiguous with the epididymal head distally and vas deferens proximally. The vas deferens is generally indistinguishable from other spermatic cord contents. The spermatic cord has the ultrasound appearance of a nonperistalsing tight bundle of tubular elements coursing from the scrotum to the internal inguinal ring, with some tubular elements having demonstrable Doppler blood flow.
As mentioned above, a transabdominal approach with ultrasound when the bladder is adequately distended with urine best shows the seminal vesicles and prostate gland in children. Like the testicles, their sizes increase during puberty. The normal prostate gland and seminal vesicles are often difficult to visualize in prepubertal children unless pathologically enlarged.
SPECTRUM OF TESTICULAR AND SCROTAL DISORDERS
Congenital and Developmental Anomalies
Cryptorchidism
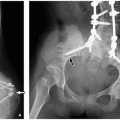
Cryptorchidism is defined as the absence of one or both testicles from the scrotum, and the incidence of congenital cryptorchidism in full-term males is estimated at 2% to 4%.7 Testicles originate in the high retroperitoneum; therefore, ultrasound evaluation for undescended testicles must extend from below the liver and spleen, inferiorly through retroperitoneum and inguinal canals, to the scrotum (Fig. 18.4). Multiple risk factors have been implicated for causing undescended testicles, including prematurity and multiple maternal factors/exposures; however, no single etiology stands out.8,9,10
If undescended testicles are occult to ultrasound, MRI may be helpful, particularly using T2-weighted and diffusion-weighted sequences where they appear hyperintense (Fig. 18.4). The undescended testicle has a higher risk of malignancy (especially seminoma), and it is unclear whether this risk is decreased with surgical repair.11
Ambiguous External Genitalia
Ambiguous external genitalia can be a source of great concern and require a multidisciplinary approach to determine the correct diagnosis. The currently accepted term for the spectrum of etiologies is “disorders of sex development” (DSD). It should be noted that not all DSD manifests as ambiguous external genitalia. In the absence of the Y chromosome, the internal and external genitalia develop the “default” female phenotype.
Ultrasound workup of ambiguous genitalia must include evaluation of scrotum/labia, inguinal canals, pelvis, kidneys, and adrenal glands. As shown in Table 18.1, identification of the uterus and characterization of both gonads are critical to classifying the DSD. The most common cause of ambiguous genitalia is congenital adrenal hyperplasia, which is one of the several causes of female pseudohermaphroditism (46,XX).12 In congenital adrenal hyperplasia, the adrenal glands are often enlarged with a “cerebriform” appearance. The most common cause of ambiguous genitalia with a 46,XX genotype is androgen insensitivity syndrome, which is one of the several causes of male pseudohermaphroditism.12 In complete androgen insensitivity (Fig. 18.4), the external genitalia are female, whereas in partial androgen insensitivity, the external genitalia are ambiguous. In some children, DSD may be very complex, requiring MRI evaluation and a systematic interdisciplinary approach to fully classify and characterize the disorder (Fig. 18.5).
Adrenal Cortical Rests/Testicular Adrenal Rest Tumors (TARTs)
Heterotopic adrenal cortical tissue can be found in the paratesticular region and sometimes within the testicle. Intratesticular adrenocortical tissue can proliferate into tumor-like masses in patients with congenital adrenal hyperplasia, and Nelson syndrome can sometimes present with detectable intratesticular masses at ultrasound both during childhood and adulthood.13,14,15 The adrenal tissue becomes trapped in the testicles in utero prior to migration from the high retroperitoneum and subsequently enlarges in response to elevated adrenocorticotropic hormone.
Although their ultrasound appearance can be variable, these rests most often present as one or more bilateral intratesticular hypoechoic masses (Fig. 18.6).13 Testicular venous sampling for elevated cortisol levels may confirm the diagnosis.16
Although their ultrasound appearance can be variable, these rests most often present as one or more bilateral intratesticular hypoechoic masses (Fig. 18.6).13 Testicular venous sampling for elevated cortisol levels may confirm the diagnosis.16
Splenogonadal Fusion
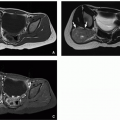
Splenogonadal fusion is a rare cause of an extratesticular mass that results from the migration of splenic tissue with the testicle.17 The connection may be direct (continuous) with the principle spleen fused to the intrascrotal testicle or indirect (discontinuous) with independent (ectopic) splenic tissue traveling into the scrotum with the testicle. Although the involved splenic tissue should have similar imaging features of native spleen at ultrasound, CT, and MRI (Fig. 18.7), there may be overlap in appearance with the testicle. Although scintigraphy (99mTclabeled sulfur colloid or damaged red blood cells) could potentially help in the diagnosis,18 splenogonadal fusion is rare; therefore, most patients go on to surgery for removal of a scrotal mass and the diagnosis is confirmed by histopathology.
TABLE 18.1 Disorders of Sex Development | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Tunica Albuginea Cyst
Tunica albuginea cysts are common benign palpable avascular cysts that arise from the tunica albuginea, the fibrous capsule surrounding the testicle. Uncomplicated cysts are anechoic at ultrasound and may exert mass effect on the underlying testicular parenchyma (Fig. 18.8). Complicated cysts may contain debris or calcification. The location of this type of cyst is key to distinguishing it from other cystic abnormalities arising from either the testicle or epididymis.19
Bell Clapper Anomaly and Testicular Torsion
If the processus (tunica) vaginalis completely envelopes the testicle and there is failure of normal posterior anchoring of the testicle to the scrotum, the resulting configuration is called a “bell clapper” anomaly. This is because the testicle can freely swing and rotate within the processus vaginalis like a bell clapper in a bell (Fig. 18.9). Normally, a broad base of direct testicular contact with the internal spermatic fascia prevents free mobility. Therefore, children with a bell clapper anomaly are at increased risk of intravaginal testicular torsion.20 The incidence of bell clapper anomaly has been reported to be 12% in autopsy series,21 although the frequency of torsion is far less than this22; therefore; other factors are likely involved.
Testicular torsion is the twisting of the testicle about the spermatic cord and causes obstructed venous outflow, lack of arterial inflow, and eventual infarction of the testicle (Fig. 18.10). The clinical presentation is most often severe acute unilateral scrotal pain, nausea, and vomiting; physical exam may reveal a high-riding horizontal testicle with an absent cremasteric reflex.23 The acute presentation should help distinguish torsion from the gradual symptom onset of epididymitis or orchitis; however, intermittent torsion may present more subacutely.24 The lack of venous outflow leads to testicular congestion (edema), and this presents at ultrasound as an enlarged testicle with decreased echogenicity and heterogeneous echotexture as well as decreased Doppler
blood flow (Fig. 18.11). Prolonged torsion may result in a markedly enlarged, heterogeneous testicle with areas of geographic increased and decreased echogenicity. However, as abnormally decreased Doppler blood flow is only 84% sensitive for detecting torsion, normal or increased intratesticular Doppler signal should not exclude the diagnosis when clinical suspicion is high.25 Presumably, a false-negative Doppler ultrasound examination is commonly due to intermittent torsion.
blood flow (Fig. 18.11). Prolonged torsion may result in a markedly enlarged, heterogeneous testicle with areas of geographic increased and decreased echogenicity. However, as abnormally decreased Doppler blood flow is only 84% sensitive for detecting torsion, normal or increased intratesticular Doppler signal should not exclude the diagnosis when clinical suspicion is high.25 Presumably, a false-negative Doppler ultrasound examination is commonly due to intermittent torsion.
 FIGURE 18.9 A: Normal posterior fixation of the testicle in the scrotum. B: Bell clapper anomaly without torsion. Note the lack of normal posterior fixation. C: Extravaginal testicular torsion without bell clapper anomaly. D: Intravaginal testicular torsion with bell clapper deformity. Note that fluid is present posterior to the testicle. (Reproduced with permission from RSNA and the copyright owner.121) |
There are two unique types of testicular torsion that occur in children. As discussed above, the bell clapper anomaly is a developmental abnormality where the testicle is inadequately affixed to the posterior scrotum, thus allowing the spermatic cord and testicle to twist within the confines of the processus (tunica) vaginalis. This type of torsion is therefore defined as intravaginal. The diagnosis of a bell clapper anomaly can be confidently made when fluid completely surrounds the testicle without the usual posterior anchoring of the gubernaculum, epididymis, and testis to the scrotum. The “torsion knot” sign may also be observed, appearing as a supratesticular mass-like abnormality, which is due to twisted, edematous spermatic cord.26 Intravaginal torsion is much more common in adolescents, whereas extravaginal torsion (described below) is more common in neonates.
Very young children (including fetuses and neonates) are susceptible to testicular torsion due to a deficient gubernaculum, which allows the testicle and processus vaginalis to twist together. This type of torsion is referred to as extravaginal, and it most often occurs in utero or soon after birth. At ultrasound, findings suggestive of extravaginal testicular infarction include a small hypoechoic testicle (frequently inguinal in location) with a calcific rim (the rim is echogenic and may show posterior acoustic shadowing) and decreased Doppler blood flow27 (Fig. 18.12).
Treatment of testicular torsion generally requires surgery; however, there is only about a 4- to 8-hour window after onset before permanent ischemic damage occurs. Delay in treatment may result in infertility or require orchiectomy.23 In cases where a bell clapper anomaly is present, bilateral orchiopexy is performed. Based on a recent survey of practicing urologists, management of neonatal (including prenatal/perinatal) torsion is inconsistent with children undergoing surgical exploration on emergent, urgent, and elective bases.28 In the setting of neonatal torsion (when bell clapper anomaly is not the primary mechanism of torsion), many surgeons also still perform bilateral orchiopexy.28,29
Infectious and Inflammatory Disorders
Orchitis
Pediatric patients with infectious or inflammation of the testicle and epididymis may present with severe pain, swelling, and hematuria and/or ejaculation of blood, and presenting signs and symptoms overlap considerably with testicular torsion.25,30,31,32,33,34 The primary cause of orchitis and epididymitis in young children is an underlying genitourinary tract abnormality (e.g., neurogenic bladder or ectopic ureter), whereas a descending or sexually transmitted urinary tract infection from E. coli, N. gonorrhea, or Chlamydia is more common in adolescents. Viruses, including mumps, are another potential infectious cause.35
Ultrasound can help differentiate between orchitis and testicular torsion. Orchitis frequently shows an enlarged, heterogeneous, hypoechoic testicle with increased color Doppler blood flow30 (Fig. 18.13), whereas torsion most often has absent or decreased Doppler blood flow. Both echotexture and Doppler blood flow can be variable in appearance with epididymo-orchtis, however: the testes and epididymides have normal echotexture and show only hyperemia in 20% of patients with epididymitis and 40% of patients with orchitis.36 An enlarged epididymis in children with testicular torsion may lead to a misdiagnosis of epididymitis in cases of partial or intermittent torsion and normal or increased testicular blood flow.37,38 The sonographic findings of leukemia or lymphoma involving the testicle show considerable overlap with and may be challenging to distinguish from epididymo-orchitis.39 Once other etiologies are excluded, the management of epididymo-orchitis is directed at the suspected organism with antibiotic therapy, hot or cold packs for analgesia, and scrotal elevation.40
Hydrocele, Pyocele, and Hematocele
Increased fluid in the scrotal sac is a common finding on ultrasound and is most often due to a simple hydrocele.30,41,42 Hydroceles are collections of fluid that develop between the visceral and parietal layers of the tunica vaginalis and/or along the spermatic cord, and at ultrasound they typically appear as thin-walled anechoic fluid collections (Fig. 18.14) or fluid collections with low-level swirling echoes43. Children with hydroceles most commonly present with painless scrotal swelling. The etiology is congenital in most neonates and infants as well as some older children, because of a patent processus vaginalis (Fig. 18.15). Hydroceles in older children and adolescents are more likely to be acquired from inflammation/infection, testicular torsion, trauma, or a tumor.30 In rare cases, very large hydroceles may communicate with the abdominal cavity, having an “hour-glass” configuration, and are called abdominoscrotal hydroceles44,45,46 (Fig. 18.16). Spermatic cord hydroceles may be classified into two types: the fluid of an encysted hydrocele does not communicate with the peritoneal cavity above or the scrotal sac below, whereas the fluid of a funicular hydrocele communicates with the peritoneal cavity at the internal ring but not the scrotum.47,48
Other cystic/fluid-filled abnormalities that may occupy the scrotal sac and inguinal canal include infection (pyocele and scrotal abscess), hematocele (usually due to recent trauma), and bowel from an inguinal hernia (Fig. 18.17). Pyoceles and hematoceles are commonly complex in appearance, containing debris, septations, and fluid-fluid levels at ultrasound.
Torsion of Intrascrotal Appendages (Appendix Testis and Appendix Epididymis)
Torsion of an intrascrotal appendage is a common cause of acute scrotal pain during the pediatric period.49 These appendages are embryonic remnants of the paramesonephric (testicular) and mesonephric (epididymis) ducts and are named based on their location.36,50 Three of five types can be identified on ultrasound and are more readily apparent in the presence of a hydrocele—the appendix testis, appendix epididymis, and appendix of the epididymal tail.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree