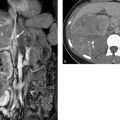
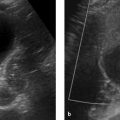
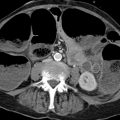
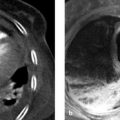
Imaging Ultrasonography, including color duplex sonography and power Doppler, is still the primary modality for imaging diseases of the testis and epididymis. MRI is a valuable adjunct that can add significant information owing to its excellent soft tissue contrast; it is used mainly to resolve discrepancies between clinical and sonographic findings. MRI is particularly helpful in the investigation of sonographically indeterminate lesions. The normal testis in an adult is oval in shape with a long-axis diameter of 3 to 5 cm and a short-axis diameter of 2 to 4 cm (▶ Fig. 13.1). It is normal for the two testes to differ slightly in size. The testis consists of approximately 250 to 400 lobules, which are compartmentalized by a radial pattern of septa. These septa extend into the testis from the outer fibrous capsule, the tunica albuginea, and coalesce to form the mediastinum testis, which also transmits branches of the testicular artery and vein. The testicular lobules are composed of multiple tortuous seminiferous tubules, which converge centrally to form the rete testis. The rete is drained by approximately 10 to 15 excretory ducts, the efferent ductules, which transport sperm from the rete testis to the epididymis. Fig. 13.1 (a) Structure of the testis and epididymis. (b) Structure of the testicular wall. (c) Blood vessels. (Reproduced from Schünke M, Schulte E, Schumacher U. Prometheus. LernAtlas der Anatomie: Innere Organe. Illustrated by M. Voll/K. Wesker. 2nd ed. Stuttgart: Thieme; 2009 and 2011.) The elongated epididymis consists mostly of the tightly coiled epididymal duct and is approximately 6 to 7 cm long. It is located on the posterolateral side of the testis and is divisible into a head, body, and tail. The head is located at the upper pole of the testis, the tail at its lower pole. The tail gives rise to the vas deferens (ductus deferens), which enters the abdomen in the spermatic cord and runs from there to the prostate. The spermatic cord also transmits the testicular artery and vein, the pampiniform plexus, and the nerves and lymphatics of the testis. The tunica vaginalis, composed of a parietal and a visceral layer, is the part of the peritoneum that preceded the descent of the testis from the abdomen into the scrotum. It forms a pouch that envelops almost all of the testis except where it connects to the epididymis. The communication between the pouch and peritoneal cavity is obliterated after birth. A small amount of fluid is normally present between the two layers of the tunica vaginalis, creating a mobile interface. The tunica vaginalis is continuous externally with the cremaster muscle, which is covered by scrotal skin. The testes are separated from each other by a fibromuscular septum derived from the subcutaneous tissue of the scrotal skin. The testis receives its blood supply from the testicular artery. Both the right and left arteries arise from the aorta just below the renal arteries. The testicular vein collects blood from the pampiniform plexus; it opens directly into the inferior vena cava on the right side and into the left renal vein on the left side. Sonographic landmarks The testis has a homogeneous, intermediate echogenicity that is interrupted only by the eccentrically placed, hyperechoic mediastinum testis (▶ Fig. 13.2). The tunica albuginea that encloses the testis appears as a thin, hyperechoic line upon the testis. The scrotal cavity, with its thin echo-free fluid rim, is most clearly visible at the head of the epididymis. The head of the epididymis, like the epididymis as a whole, is isoechoic or slightly hypoechoic and has a slightly coarser echo texture than the testis. Differentiation of the testis and epididymis is most easily accomplished in longitudinal scans. Fig. 13.2 Normal ultrasound appearance of the testis. (a) Normal testis of moderate echogenicity, viewed in longitudinal scan. Note the hyperechoic tunica albuginea bordered by a small amount of hypoechoic fluid between the parietal and visceral layers of the tunica vaginalis (arrow). The wedge-shaped feature at upper left is the head of the epididymis (triangle), which is less echogenic than the testis and shows a slightly coarser texture. (b) This scan displays the tail of the epididymis (star) and the hyperechoic mediastinum testis (arrow), which occupies an eccentric, posterolateral position. MRI landmarks The testes are hyperintense on T2W images and has homogeneous intermediate signal intensity on T1W images (▶ Fig. 13.3). The tunica albuginea is most clearly depicted on T2W images as a thin, hypointense band surrounding the testis. The eccentrically placed mediastinum testis is also hypointense on T1W and T2W images (▶ Fig. 13.4). The epididymis is slightly more heterogeneous than the testis, appearing hypo- to isointense on T1W images and hypointense on T2W images. The epididymis enhances more than the testis after IV administration of contrast medium. Fig. 13.3 Normal MRI appearance of the testis. Parasagittal images. (a) T2W image. The testicular parenchyma appears hyperintense. The tunica albuginea enclosing the testis appears as a linear hypointensity (thin arrow). The mediastinum testis with the rete testis (thick arrow) forms a hypointense connection between the testis and epididymis. The head of the epididymis (star), tail of the epididymis (triangle), and spermatic cord (circle) are clearly visualized. (b) T1W image. The testis and epididymis are poorly differentiated from each other on T1W images, as both appear hypointense. Fig. 13.4 Normal MRI appearance of the testis. The septa permeating the testis and the mediastinum testis (arrow) appear hypointense in this paracoronal T2W image. The head of the epididymis (triangle) and the spermatic cords (circles) in the inguinal canal are also clearly displayed. The medial oval structures are the corpora cavernosa of the penis. Brief definition Normally the testis descends through the inguinal canal and into the scrotum during the 36th week of gestation. Cryptorchidism is a condition in which the testis remains undescended or maldescended, causing it to be absent from the scrotum. An incompletely descended testis is usually within the inguinal canal and will complete its descent during the first year of life. An undescended testis may also be located within the abdomen or at ectopic sites outside the normal track of descent. Imaging signs The undescended testis appears small and hypoechoic on ultrasound (▶ Fig. 13.5). If the testis is not found during ultrasound scanning, it should be located by MRI or, alternatively, by laparoscopy. On MRI the undescended testis is hypointense in the T1W sequence and hyperintense in the T2W sequence. If the testis is atrophic, it will show decreased signal intensity on T2W images. An undescended testis often shows little or no enhancement after IV administration of contrast medium. Fig. 13.5 Cryptorchidism (undescended testis). (a) Ultrasound B-mode image reveals the small, hypoechoic undescended testis (arrow) in the inguinal canal at the level of the internal (superficial) inguinal ring. (b) Corresponding laparoscopic view. Clinical features Failure of a testicle to descend during the first year of life may result in infertility because the intra-abdominal body temperature is higher than the scrotal temperature. This temperature differential also increases the risk of developing testicular cancer. Pitfalls The undescended testis may be misinterpreted as a lymph node. Key points Cryptorchidism refers to a maldescended or undescended testis. The testis is often located in the inguinal canal, where it is easily identified with ultrasound. An intra-abdominal testis is detectable by laparoscopy or MRI. Cryptorchidism is associated with an increased risk of testicular cancer. Brief definition A varicocele is formed by dilated, tortuous veins in the pampiniform plexus. Idiopathic varicocele results from incompetent venous valves and is a frequent cause of infertility. A secondary varicocele is caused by hampered venous return in the testicular vein due to a rise of retroperitoneal pressure (e.g., retroperitoneal mass or hydronephrosis) or inferior vena cava thrombosis. Imaging signs Ultrasound scans should be performed in both the standing and supine positions and should include a Valsalva maneuver. The probe can be moved down along the spermatic cord to the pampiniform plexus. Scans at that level will demonstrate multiple dilated, tortuous veins more than 2 to 3 mm in diameter in the spermatic cord and around the epididymis. Blood flow in the affected area is very slow and can be detected by color duplex sonography at sensitive settings. Idiopathic varicoceles tend to drain when the patient is positioned supine, whereas secondary varicoceles persist; MRI does not have a role in making this determination. The internal spermatic vein is classified into different anatomical types based on the presence of collateral vessels to renal capsular vessels, lumbar veins, or the inferior vena cava. The collaterals are visualized during interventional therapy by injecting contrast material into the left renal vein or by selective catheterization. The presence of collaterals will determine the risk of reflux of sclerosant and the risk of recurrence after interventional sclerotherapy. Clinical features Most varicoceles are asymptomatic and are often detected incidentally in a morphologically normal scrotum. They are more common on the left side because the testicular vein drains at a 90° angle into the left renal vein. Symptomatic patients may complain of tension or position-dependent pain that is exacerbated by standing or walking. These cases can be treated by interventional sclerotherapy with access through the (right) common femoral vein and selective catheterization of the left testicular vein. Pitfalls The varicocele may be missed if the examination is performed only in the supine position and/or without a Valsalva maneuver. Differential diagnosis The clinical differential diagnosis includes hydroceles, spermatoceles, hernias, and testicular tumors. These conditions are easily differentiated from one another by imaging. Key points Varicoceles are caused by dilated, tortuous veins in the pampiniform plexus. They are diagnosed by B-mode ultrasound imaging and color duplex scanning in both the standing and supine positions, aided by a Valsalva maneuver. The diagnosis of a secondary varicocele requires the exclusion of a retroperitoneal mass (e.g., renal tumor). Brief definition Supravaginal testicular torsion, which is more common in newborns, is distinguished etiologically from intravaginal torsion of the spermatic cord, which is much more common in adolescents and adults. A predisposing factor for intravaginal torsion is the “bell clapper” deformity, in which the tunica vaginalis completely surrounds the testis, allowing the spermatic cord to become twisted on its longitudinal axis. The degree of spermatic cord torsion dictates the severity of decreased arterial blood flow. Interruption of venous drainage leads to hemorrhagic infarction of the testicular parenchyma. Imaging signs Both sides should be imaged sonographically and the affected testis compared with the nonpainful side. In the acute stage of testicular torsion (the first 6 hours), the testis is enlarged and has a heterogeneous, hypoechoic appearance. The epididymis is also enlarged and is heterogeneously hypoechoic. A thin hydrocele may additionally be present. In cases where the torsion is undiagnosed, the subacute stage (the next 5 days) is marked by increasing enlargement of the testis and epididymis, which exhibit a more heterogeneous echo pattern. Unless the torsion is reduced, the testis will become atrophic and show decreasing echogenicity. Absence of flow signal in the affected testis on color duplex sonography or power Doppler confirms the diagnosis of testicular torsion (▶ Fig. 13.6). Milder degrees of torsion (less than 360°) may initially compromise only venous flow in the affected testis while arterial blood flow is still detectable, although it is often diminished relative to the opposite side. Fig. 13.6 Testicular torsion. Power Doppler image shows a hypoechoic testis that is devoid of flow signals and occupies an abnormally high position. The absence of detectable blood flow confirms testicular torsion. The hypoechoic testicular parenchyma indicates an older process. Clinical features Testicular torsion presents with acute pain of sudden onset in the affected testis or scrotum. Pitfalls Detectable blood flow in the affected testis does not exclude testicular torsion, especially if it is decreased relative to the opposite side. Note In doubtful cases where testicular torsion is suspected but blood flow is present, the testis should be surgically exposed. Differential diagnosis After acute epididymitis or epididymo-orchitis, testicular torsion is the second most common cause of acute testicular or scrotal pain. Absence of flow signal in the affected testis confirms testicular torsion, while increased blood flow is suggestive of epididymo-orchitis. Another differential diagnosis is torsion of the testicular or epididymal appendix. Key points Testicular torsion is marked by acute onset of severe pain in the testis or scrotum. The cause is torsion of the spermatic cord about its long axis, compromising arterial blood flow to the testis. The testis may be devoid of flow signal by color duplex and power Doppler ultrasonography, or milder degrees of torsion may cause diminished flow relative to the opposite side. Brief definition Inflammations of the epididymis and testis are the most frequent cause of acute testicular and scrotal pain. Acute epididymitis very often results from an ascending bacterial infection of the lower urinary tract. It usually affects the head of the epididymis, although the entire epididymis may be involved. Isolated orchitis may occur in a setting of mumps. Imaging signs Sonographically, the affected portions of the epididymis are enlarged and hypoechoic to normal surrounding tissue. Color duplex and power Doppler ultrasonography show increased blood flow (▶ Fig. 13.7). If the testis is also affected (acute epididymo-orchitis), it appears enlarged and shows increased blood flow by color duplex and power Doppler ultrasonography. Its echo pattern in the B-mode image is heterogeneously hypoechoic. In more severe cases of epididymo-orchitis, the inflammatory process may give rise to a testicular abscess. Ultrasound in this case will show a heterogeneous fluid collection with a hypervascular rim within the enlarged testis. On MRI, the affected epididymis is enlarged and shows a heterogeneous, predominantly hyperintense signal pattern on T2W images. Cases complicated by intrascrotal hemorrhage show variable T1W and T2W signal characteristics depending on the age of the hemorrhage. Inflamed areas enhance intensely after administration of gadolinium contrast. In the case of orchitis, affected areas show homogeneous to heterogeneous hyperintensity on T2W images. Fig. 13.7 Epididymitis. Longitudinal ultrasound scan of the testis shows an enlarged epididymal head (triangle) with increased color flow signals. Clinical features Epididymitis and epididymo-orchitis present clinically with increasing pain that is exacerbated by pressure and movement. Physical lifting of the testis relieves pain due to epididymitis (positive Prehn’s sign). Fever and voiding problems may also be present. Differential diagnosis Differentiation is required mainly from testicular torsion. In contrast to epididymitis, increased blood flow is not seen on color duplex sonography. A testicular tumor may cause diagnostic confusion, especially in patients with chronic inflammatory testicular changes. Key points Inflammatory changes in the testis and epididymis account for the majority of acute, painful scrotal diseases. On ultrasound the affected areas are enlarged and heterogeneously hypoechoic. Color duplex and power Doppler ultrasonography show increased blood flow. Brief definition The testis is susceptible to injury by blunt or penetrating trauma. Testicular rupture constitutes a urological emergency, in which ultrasound plays a key diagnostic role. Imaging signs The ruptured testis has indistinct margins and an irregular contour. Sonographically, the testicular parenchyma shows heterogeneous echogenicity as a result of intratesticular hemorrhage and infarction. Extratesticular blood collections (epididymal hemorrhage, hematocele) may hinder the examination. Switching to color duplex and/or power Doppler mode during the examination is very helpful in distinguishing between vascularized testicular parenchyma and nonvascularized hematoma. MRI is excellent for evaluating the integrity of the tunica albuginea on T2W images. Additionally, the injured testis appears hypointense to the uninjured side on T2W images and shows less enhancement on T1W images after administration of gadolinium contrast medium. The appearance of intratesticular hematomas depends on the age of the collection. Acute hematomas show decreased signal intensity on T1W images and increased signal intensity on T2W images. Chronic hematomas, on the other hand, are hyperintense in both T1W and T2W sequences. Clinical features The testis is painful and the scrotal skin shows livid discoloration. Extensive hematomas are most often found in association with blunt trauma. Pitfalls A common error is to omit daily ultrasound follow-ups of hematomas that have not been surgically evacuated. Key points The testis may be injured as a result of acute or blunt trauma. The initial imaging study of choice is ultrasonography. The affected testis shows a heterogeneous echo pattern due to intratesticular hemorrhage and infarction. Extratesticular hematomas are a common associated finding. MRI is best for assessing the integrity of the tunica albuginea. Brief definition Hydrocele is a collection of excessive fluid (small amounts are normal) between the parietal and visceral layers of the tunica vaginalis and is the most common cause of testicular swelling. Congenital hydrocele results from persistent patency of the vaginal process; this type will resolve as the process obliterates during the first year of life. Acquired hydroceles may result from prior trauma, epididymitis or epididymo-orchitis, inguinal hernia, or a testicular tumor. Acquired hydroceles may also be idiopathic. Imaging signs Idiopathic hydrocele is composed of serous fluid that appears echo-free on ultrasound (▶ Fig. 13.8). This differs from traumatic hematocele and pyocele in a setting of acute epididymitis or epididymo-orchitis, which are hypoechoic and contain echogenic septa and protein precipitates that create internal echoes. On MRI, hydroceles are diagnosed in association with other testicular diseases and show typical fluid signal characteristics (hypointense in T1W sequences, hyperintense in T2W sequences). MRI is not used for the initial diagnosis of hydroceles. Testicular and epididymal appendices are most clearly visualized when a hydrocele is present. They are embryonic remnants that are normally only a few millimeters long and may be found at the upper pole of the testis or epididymis. Fig. 13.8 Testicular hydrocele. With a hydrocele (stars), the normal testis is surrounded by an abnormally increased volume of serous fluid. Clinical features Hydroceles are painless and may gradually increase in size. The enlargement may be perceived as a sensation of pressure or heaviness. Pitfalls A hydrocele with internal septa may be mistaken for a spermatocele in the B-mode image. Differential diagnosis Pyoceles and hematoceles have internal echoes and sometimes contain septa. Color duplex and power Doppler scans show the presence of flow signals in spermatoceles. Key points Hydrocele is a painless fluid collection between the two layers of the tunica vaginalis. The imaging modality of choice is ultrasound, which shows an echo-free fluid collection devoid of flow signals. Acquired hydroceles contain internal echoes caused by precipitated proteins and are often septated. Brief definition Spermatoceles are located within the head of the epididymis. They may result from prior trauma or epididymitis. Spermatocele is composed of protein-rich fluid with cellular breakdown products. Imaging signs A spermatocele appears sonographically as a well-circumscribed, echo-free to hypoechoic mass with internal echoes and posterior acoustic enhancement (▶ Fig. 13.9). On MRI it displays typical cystlike fluid characteristics (hypointense in T1W sequences, hyperintense in T2W sequences; ▶ Fig. 13.10). Fig. 13.9 Large spermatocele. (a) Composite longitudinal ultrasound scans of a spermatocele (diamonds) permeating the entire epididymis. The scans include sections of normal testis (circles) on the ipsilateral and contralateral sides. (b) Magnified view displays the hypoechoic cystic structures with internal echoes. Fig. 13.10 Spermatocele in the head of the epididymis. T2W images of a spermatocele in the right epididymal head ([a], [b], open arrows). A smaller spermatocele in the left epididymal head ([a], arrow) is also shown. (a) Coronal T2W image. (b) Sagittal T2W image. Clinical features Spermatoceles do not cause complaints. They are often noted as incidental findings. Spermatoceles may be associated with a dull aching sensation. Differential diagnosis Simple epididymal cyst: This lesion is less common than spermatocele and may occur anywhere in the epididymis. It is echo-free on ultrasound and shows posterior acoustic enhancement. On MRI it presents smooth margins and is hypointense in T1W sequences and hyperintense in T2W sequences (▶ Fig. 13.11). Testicular and tunica cysts: A testicular cyst is located within the testis close to the mediastinum, while a tunica cyst is located in the tunica albuginea (▶ Fig. 13.12). Fig. 13.11 Simple epididymal cyst. Typical appearance of a simple cyst in the epididymal head ([a], [b], arrows). The well-circumscribed cyst is hyperintense in the T2W image (a) and hypointense in the T1W image (b). A hydrocele is also present. (a) T2W image. (b) T1W image. Fig. 13.12 Testicular cyst and tunica cyst. The testicular cyst ([a], [b], open arrows) and tunica cyst ([a], [b], arrows) show typical high signal intensity in the T2W image (a) and low signal intensity in the T1W image (b). (a) Axial T2W image. (b) Axial T1W image. Key points A spermatocele is composed of protein-rich fluid and is located in the epididymal head. It presents smooth margins. Sonographically it is echo-free to hypoechoic with internal echoes and shows posterior acoustic enhancement. The differential diagnosis includes simple epididymal cysts as well as testicular and tunica cysts. Brief definition Testicular microlithiasis is defined as the presence of multiple microcalcifications in a normal or undescended testis. The microcalcifications are 1 to 2 mm in size and are located in the seminiferous tubules of the testicular lobules. The pathogenesis of testicular microlithiasis is not fully understood, but it is currently believed that the calcifications result from cellular necrosis. Testicular microcalcifications are a predisposing factor for the development of testicular cancer. Consequently, these patients should be scheduled for regular ultrasound follow-ups and laboratory tests for tumor markers. Imaging signs Ultrasound shows multiple 1- to 2-mm hyperechoic lesions distributed diffusely in the testicular parenchyma (▶ Fig. 13.13). MRI aids in the detection or exclusion of suspicious focal lesions revealed by ultrasound. Fig. 13.13 Testicular microlithiasis. The diffusely distributed microcalcifications in testicular microlithiasis appear sonographically as small hyperechoic foci (arrows) in the testicular parenchyma, which shows normal echogenicity. Clinical features Testicular microlithiasis is an incidental finding and does not cause complaints. Pitfalls A common error is failure to maintain follow-up in patients with microlithiasis. Differential diagnosis Differentiation is mainly required from microcalcifications associated with testicular tumors or previous trauma (▶ Fig. 13.14). Fig. 13.14 Extratesticular macrocalcification. A macrocalcification (hexagon) is found outside the testis (circle) following trauma with an associated hematoma. An acoustic shadow (arrow) is present posterior to the macrocalcification. Key points Ultrasound in patients with testicular microlithiasis shows multiple, diffusely distributed echogenic lesions 1 to 2 mm in size (microcalcifications). Note Microlithiasis has a strong association with testicular tumors and these patients should be scheduled for regular ultrasound follow-ups. Brief definition Testicular tumors are subdivided into three main groups: Germ cell tumors (ca. 90% of all testicular tumors). Sex cord-stromal tumors (ca. 5% of all testicular tumors). Lymphomas and metastases. They are the most common tumors in males 20 to 40 years of age. Three different classifications are currently used for the description of testicular tumors and their metastasis: TNM classification (▶ Table 13.1.) Lugano classification for staging and response assessment (▶ Table 13.2). Classification of the International Germ Cell Cancer Collaborative Group (IGCCCG) for defining prognostic groups. Designation Description Primary tumor Tx Primary tumor cannot be assessed T0 No evidence of a primary tumor (e.g., if only scar is found in the testis, same as burned-out tumor) Tis Intratubular germ cell neoplasia (carcinoma in situ) T1 Tumor limited to the testis and epididymis T2 Tumor limited to the testis and epididymis, with involvement of lymphatics or blood vessels of the tunica vaginalis T3 Tumor invades the spermatic cord T4 Tumor invades the scrotum Regional lymph nodes Nx Regional lymph nodes cannot be assessed N0 No regional lymph node metastasis N1 Metastasis with a lymph node mass ≤2 cm in greatest dimension and ≤5 nodes positive N2 Metastasis with a lymph node mass >2 cm but ≤5 cm in greatest dimension; or >5 nodes positive, none >5 cm N3 Metastasis with a lymph node mass >5 cm in greatest dimension Distant metastasis M0 No distant metastasis M1 Distant metastasis Serum tumor markers Sx Marker studies not available S0 Marker study levels within normal limits S1 LDH <1.5 times normal and β-HCG <5,000 mIU/mL and AFP <1,000 ng/mL S2 LDH 1.5–10 times normal or β-HCG 5,000–50,000 mIU/mL or AFP 1,000–10,000 ng/mL S3 LDH >10 times normal or β-HCG >50,000 mIU/mL or AFP >10,000 ng/mL Abbreviations: AFP, alpha-fetoprotein; hCG, human chorionic gonadotropin; LDH, lactate dehydrogenase. Stage Description I No evidence of metastases IIA Retroperitoneal lymph node metastases <2 cm IIB Retroperitoneal lymph node metastases 2–5 cm IIC Retroperitoneal lymph node metastases >5 cm III Supradiaphragmatic lymph node metastases and/or hematogenous metastases Clinical features Testicular tumors present clinically as a firm, painless testicular mass that slowly enlarges over time and may be accompanied by a hydrocele. Bleeding into the tumor or tunica is associated with acute pain. Hormone-secreting tumors may lead to gynecomastia, premature virilization, and decreased libido. Pitfalls The presence of a hydrocele may direct attention away from a possible coexisting tumor. Differential diagnosis Testicular tumors require differentiation from focal orchitis. The testis in patients with focal orchitis contains solitary or multiple hypoechoic areas. The chronic form in particular is difficult to distinguish from a testicular tumor. Infarction in the setting of epididymo-orchitis with associated hemorrhage may likewise appear as a heterogeneous hypoechoic area. The radiological differential diagnosis also includes abscesses and granulomas. Most testicular tumors measuring 1.5 cm or more in diameter will show increased blood flow by Doppler ultrasound. While this method cannot provide a more specific tumor diagnosis, it can clearly differentiate a vascularized testicular tumor from an avascular hematoma. This is particularly important in patients with a known trauma history. Brief definition Seminoma is the most common cell type (40% of testicular tumors), with a peak incidence between 30 and 50 years of age. Seminomas are round to oval-shaped tumors and may have smooth or ill-defined margins. Imaging signs When imaged with ultrasound, seminomas are homogeneously hypoechoic to surrounding testicular tissue (▶ Fig. 13.15). They may contain cysts. Color duplex and power Doppler sonography show a heterogeneous flow signal within the tumor. On MRI, these tumors typically show homogeneous low signal intensity on T2W images and are isointense on T1W images. Heterogeneous areas within the seminoma are due to regressive changes (e.g., necrosis). Seminomas show inhomogeneous enhancement after administration of gadolinium contrast medium. Neither ultrasound nor MRI can supply an accurate histological classification or positively distinguish seminomas from nonseminomatous tumors and sex cord-stromal tumors. Fig. 13.15 Seminoma. Ultrasound depicts a homogeneously hypoechoic mass (arrow) in the testis with testicular microlithiasis, which is a predisposing factor for testicular cancer.
13.1.2 Anatomy




13.1.3 Congenital Disorders: Cryptorchidism (Undescended Testis)

13.1.4 Vascular Diseases
Varicocele
Testicular Torsion

13.1.5 Inflammatory Diseases: Epididymitis and Epididymo-orchitis

13.1.6 Traumatic Disorders: Testicular Trauma
13.1.7 Benign Intrascrotal Masses
Hydrocele

Spermatocele




Testicular Microlithiasis


13.1.8 Malignant Neoplasms
Testicular Tumors
Germ Cell Tumors
Seminoma

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree