CHAPTER 3 Often cortical bone is replaced by part of the noncalcified matrix (fibrous matrix or chondroid matrix) of benign fibro-osseous lesions and cartilaginous lesions. This can give the false impression of cortical destruction on plain films (Figure 3-1) or computed tomography (CT). Also, benign processes such as infection and eosinophilic granuloma can cause extensive cortical destruction and mimic a malignant tumor. Aneurysmal bone cysts are known to cause such thinning of the cortex as to make it radiographically undetectable (Figure 3-2). For these reasons I find cortical destruction to occasionally be misleading. Cortical destruction makes one think of a malignant lesion when using the Gestalt approach, but the lesion must also have other criteria for a malignant process, such as a wide zone of transition. FIGURE 3-1 FIGURE 3-2 Periosteal reaction occurs in a nonspecific manner whenever the periosteum is irritated, whether it is irritated by a malignant tumor, a benign tumor, infection, or trauma. Callus formation in a fracture is actually just periosteal reaction of the most benign type. Periosteal reaction occurs in two types: benign (Figure 3-3, A and B) and aggressive (Figure 3–3, C and D). The difference between the two is based more on the timing of the growth of the irritation than on whether the process causing the periostitis is malignant or benign. For example, a slow-growing benign tumor will cause thick, wavy, uniform, or dense periostitis because it is a low-grade chronic irritation that gives the periosteum time to lay down thick new bone and remodel into more normal cortex. A malignant tumor causes a periosteal reaction that is high grade and more acute; hence, the periosteum does not have time to consolidate. It appears lamellated (onion skinned) or amorphous, or it may even appear sunburst. If the irritation stops or diminishes, the aggressive periostitis will solidify and appear benign. Therefore, when periostitis is seen, the radiologist should try to characterize it into either a benign (thick, dense, wavy) type or an aggressive (lamellated, amorphous, sunburst) type. FIGURE 3-3 The zone of transition is the border between the lesion and the normal bone. It is said to be narrow if it is so well defined that it can be drawn with a fine-point pen (Figure 3-4). If it is imperceptible and cannot be clearly drawn, it is said to be wide (Figure 3-5). Obviously all shades of gray lie in between, but most lesions can be characterized as having either a narrow or a wide zone of transition. If the lesion has a sclerotic border, it, by definition, has a narrow zone of transition. FIGURE 3-4 FIGURE 3-5 If a lesion has a narrow zone of transition, it is a benign process. The exceptions to that are rare, and I’m willing to miss them. If a lesion has a wide zone of transition, it is aggressive. Notice that I said aggressive and not malignant. As with aggressive periostitis, many benign lesions can have a wide zone of transition. A few of the same processes that can cause aggressive periostitis, and thereby mimic a malignant tumor, can have a wide zone of transition (i.e., infection and eosinophilic granuloma). They are aggressive in their radiographic appearance because they are fast-acting, aggressive lesions. The zone of transition is usually easier to characterize than the periostitis, plus it is always there to evaluate (or you wouldn’t see a lesion), whereas many lesions, benign and malignant, have no periostitis. For these reasons the zone of transition is the most useful indicator of whether a lesion is benign or malignant. A lesion that consists of multiple small holes is said to be permeative. It has no perceptible border and therefore has a wide zone of transition. Round cell tumors such as multiple myeloma, primary lymphoma of bone (formerly called reticulum cell sarcoma), and Ewing’s sarcoma are typical of this type of lesion. However, infection and eosinophilic granuloma (Figure 3-6) can have the same appearance. FIGURE 3-6 It is critical to be aware that the zone of transition is a plain film finding and cannot be used with magnetic resonance imaging (MRI). Many malignant tumors will appear to have narrow zones of transition on MRI examinations and can mislead one into thinking a benign lesion is present (Figure 3-7). The zone of transition can be used only with lytic lesions and on plain films. FIGURE 3-7 Once it has been decided that a particular lesion is probably malignant, the differential is fairly straightforward. First, the list of malignant tumors is relatively short, and second, most tumors follow somewhat strict age groupings. Jack Edeiken, a famed skeletal radiologist, evaluated 4000 malignant bone tumors and found that they could be correctly diagnosed 80% of the time by using only the patient’s age! He basically divides the tumors into decades of when they will affect a patient; for example, osteosarcoma and Ewing’s sarcoma are the only childhood primary malignant tumors of bone, and after the age of 40, only metastatic disease (mets), myeloma, and chondrosarcoma are common (Table 3-1). Although there are certainly outliers that are uncommon, these age guidelines are extremely useful. It is inappropriate to mention Ewing’s sarcoma in a 40-year-old or mets in a 15-year-old. Obviously, if a 15-year-old has a known primary tumor, then mets must be considered—in fact, any bone lesion, regardless of its appearance, could be a met and would be suspicious in a patient with a known primary tumor. TABLE 3-1 Malignant Tumors and Patient Age The most common malignant primary bone tumor is an osteosarcoma. Although it typically occurs toward the end of a long bone, it may occur anywhere in the skeleton with enough frequency that location is not a helpful discriminator. Osteosarcomas are usually destructive, with obvious sclerosis present from either tumor new bone or reactive sclerosis (Figures 3-8 and 3-9); however, on occasion they can be entirely lytic (Figure 3-10). These are usually so-called telangiectatic osteosarcomas. There are many types and classifications of osteosarcomas, but it serves little purpose to the radiologist to try to distinguish between most of them. FIGURE 3-8 FIGURE 3-9
Malignant tumors
Differentiation of malignant from benign
Cortical destruction
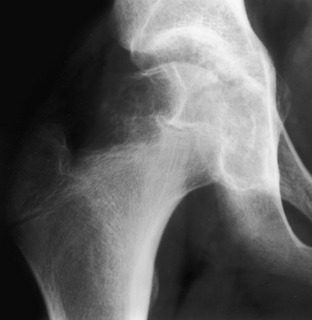
 Apparent cortical destruction. This benign chondroblastoma has noncalcified chondroid tissue replacing cortical bone in the proximal femur, which gives the lesion a destructive appearance. This is an example of cortical replacement rather than of cortical destruction, which can be very confusing if one uses cortical destruction as an aggressive or malignant key in differential diagnosis. Note that the zone of transition is narrow, as one would expect in a benign lesion such as this.
Apparent cortical destruction. This benign chondroblastoma has noncalcified chondroid tissue replacing cortical bone in the proximal femur, which gives the lesion a destructive appearance. This is an example of cortical replacement rather than of cortical destruction, which can be very confusing if one uses cortical destruction as an aggressive or malignant key in differential diagnosis. Note that the zone of transition is narrow, as one would expect in a benign lesion such as this.
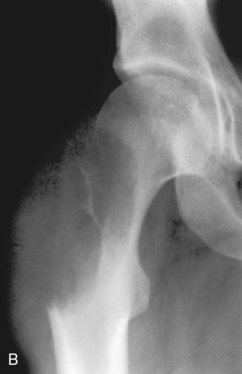
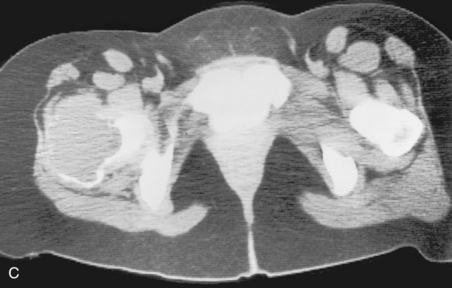
 A, Aneurysmal bone cyst. This benign lesion has thinned the cortex to such a degree as to make it imperceptible on a plain radiograph. As in Figure 3-1, this can be misconstrued as cortical destruction, giving the false impression of a malignant or very aggressive lesion. B, Giant cell tumor. This lesion in the proximal femur has expanded and thinned the cortex to such a degree that it is imperceptible on the plain film. C, On a CT scan through this region a very thin cortex that was greatly expanded was identified. This giant cell tumor originated from the greater trochanter, which is an epiphyseal equivalent. Note that it has a well-defined but nonsclerotic zone of transition, as all giant cell tumors in long bones do.
A, Aneurysmal bone cyst. This benign lesion has thinned the cortex to such a degree as to make it imperceptible on a plain radiograph. As in Figure 3-1, this can be misconstrued as cortical destruction, giving the false impression of a malignant or very aggressive lesion. B, Giant cell tumor. This lesion in the proximal femur has expanded and thinned the cortex to such a degree that it is imperceptible on the plain film. C, On a CT scan through this region a very thin cortex that was greatly expanded was identified. This giant cell tumor originated from the greater trochanter, which is an epiphyseal equivalent. Note that it has a well-defined but nonsclerotic zone of transition, as all giant cell tumors in long bones do.
Periostitis
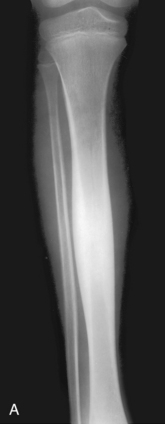
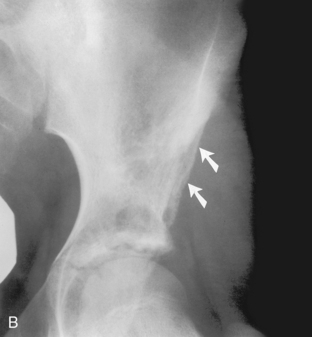
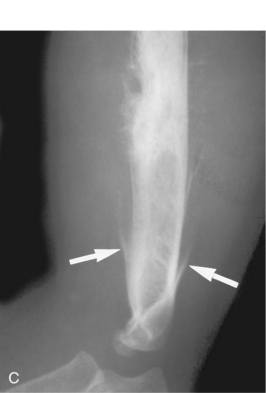
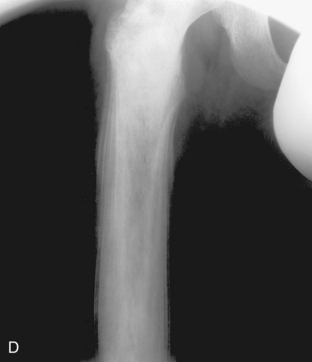
 Benign periostitis. A, An osteoid osteoma in the midshaft of the tibia has caused thick, wavy, dense periostitis, which is classic for benign type of periostitis. Malignant lesions are incapable of forming this type of periostitis and should not be considered in the differential. This type of periostitis is basically indistinguishable from callus formation in a fracture. B, Thick, wavy periostitis (arrows) along the ilium in a child with a permeative lesion in the pelvis is characteristic for infection or eosinophilic granuloma. Ewing’s sarcoma was initially considered in the differential; however, the benign periostitis would make a malignant lesion very unlikely. Biopsy showed this lesion to be eosinophilic granuloma. C, Aggressive periostitis. Amorphous, sunburst periostitis with a Codman’s triangle (arrows) in a patient with a mixed lytic-sclerotic lesion of the humerus, which on biopsy was shown to be Ewing’s sarcoma. Although this type of periostitis is characteristic for a malignant lesion, it could also be seen with benign processes such as eosinophilic granuloma or infection. D, Lamellated, or onion-skinned, periostitis is characteristic of an aggressive process, such as in this patient with Ewing’s sarcoma of the femur. Again, this aggressive type of periostitis could conceivably occur in a benign process such as infection or eosinophilic granuloma.
Benign periostitis. A, An osteoid osteoma in the midshaft of the tibia has caused thick, wavy, dense periostitis, which is classic for benign type of periostitis. Malignant lesions are incapable of forming this type of periostitis and should not be considered in the differential. This type of periostitis is basically indistinguishable from callus formation in a fracture. B, Thick, wavy periostitis (arrows) along the ilium in a child with a permeative lesion in the pelvis is characteristic for infection or eosinophilic granuloma. Ewing’s sarcoma was initially considered in the differential; however, the benign periostitis would make a malignant lesion very unlikely. Biopsy showed this lesion to be eosinophilic granuloma. C, Aggressive periostitis. Amorphous, sunburst periostitis with a Codman’s triangle (arrows) in a patient with a mixed lytic-sclerotic lesion of the humerus, which on biopsy was shown to be Ewing’s sarcoma. Although this type of periostitis is characteristic for a malignant lesion, it could also be seen with benign processes such as eosinophilic granuloma or infection. D, Lamellated, or onion-skinned, periostitis is characteristic of an aggressive process, such as in this patient with Ewing’s sarcoma of the femur. Again, this aggressive type of periostitis could conceivably occur in a benign process such as infection or eosinophilic granuloma.
Zone of transition
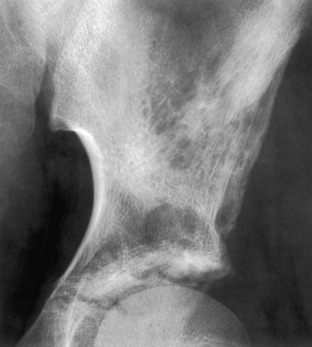
 Narrow zone of transition. The border of normal bone with a lesion is known as the zone of transition. When, as in this example of a nonossifying fibroma, the border can be drawn with a fine-point pen it is said to be a narrow zone of transition, which is characteristic of a benign lesion. A narrow zone of transition may or may not have a sclerotic border.
Narrow zone of transition. The border of normal bone with a lesion is known as the zone of transition. When, as in this example of a nonossifying fibroma, the border can be drawn with a fine-point pen it is said to be a narrow zone of transition, which is characteristic of a benign lesion. A narrow zone of transition may or may not have a sclerotic border.
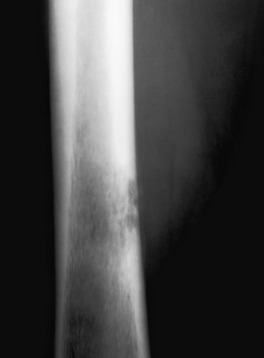
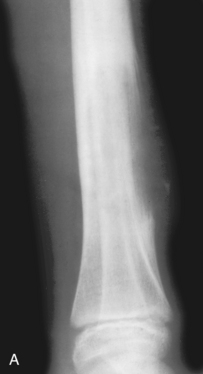
 Wide zone of transition. A lytic permeative process is seen in the midshaft of the femur in this patient. On biopsy it was found to be a malignant fibrous histiocytoma. The zone of transition in this lesion is said to be wide, because it cannot be easily drawn with a fine-point pen. A permeative lesion such as this, by definition, has a wide zone of transition.
Wide zone of transition. A lytic permeative process is seen in the midshaft of the femur in this patient. On biopsy it was found to be a malignant fibrous histiocytoma. The zone of transition in this lesion is said to be wide, because it cannot be easily drawn with a fine-point pen. A permeative lesion such as this, by definition, has a wide zone of transition.
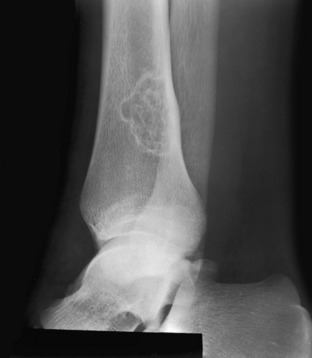
 Permeative pattern. A permeative pattern is defined as multiple, small, irregular holes in bone and indicates an aggressive process. Ewing’s sarcoma typically has a permeative pattern; however, infection and eosinophilic granuloma, as in this example, can also have a permeative pattern. This is a fine-detailed film of the same case shown in Figure 3–3, B.
Permeative pattern. A permeative pattern is defined as multiple, small, irregular holes in bone and indicates an aggressive process. Ewing’s sarcoma typically has a permeative pattern; however, infection and eosinophilic granuloma, as in this example, can also have a permeative pattern. This is a fine-detailed film of the same case shown in Figure 3–3, B.
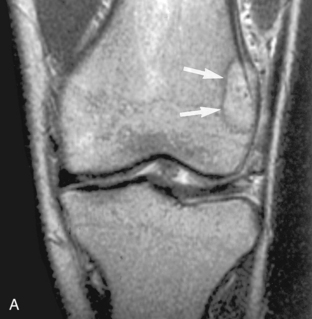
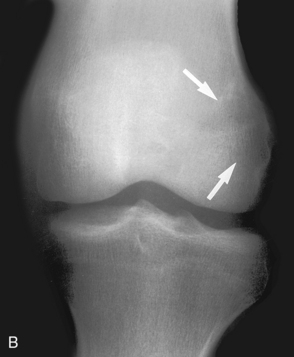
 Zone of transition on MRI. A, A T2-weighted MRI scan of the knee in this child with knee pain shows a well-defined lesion (arrow) with a low signal border, suggesting a narrow zone of transition with a sclerotic border. Because the knee pain was described as probably related to the menisci, this lesion was believed to be an incidental, benign process, such as a nonossifying fibroma. However, the plain film (B), obtained later, shows a barely discernible lytic lesion with a wide zone of transition (arrow). This lesion was, in fact, painful, and the differential diagnosis, based solely on the plain film, would include osteosarcoma—the eventual histologic diagnosis. The zone of transition can be used only on plain films; it is invalid on MRI scans.
Zone of transition on MRI. A, A T2-weighted MRI scan of the knee in this child with knee pain shows a well-defined lesion (arrow) with a low signal border, suggesting a narrow zone of transition with a sclerotic border. Because the knee pain was described as probably related to the menisci, this lesion was believed to be an incidental, benign process, such as a nonossifying fibroma. However, the plain film (B), obtained later, shows a barely discernible lytic lesion with a wide zone of transition (arrow). This lesion was, in fact, painful, and the differential diagnosis, based solely on the plain film, would include osteosarcoma—the eventual histologic diagnosis. The zone of transition can be used only on plain films; it is invalid on MRI scans.
Differentiating types of tumors
Age (yr)
Tumor
1–30
Ewing’s sarcoma
Osteosarcoma
30–40
Fibrosarcoma and malignant fibrous histiocytoma
Malignant giant cell tumor
Primary lymphoma of bone
Parosteal sarcoma
40+
Mets
Myeloma
Chondrosarcoma
Osteosarcoma
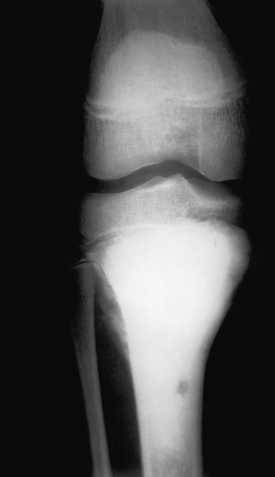
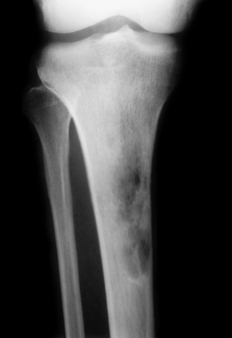
 Osteosarcoma. An extremely sclerotic lesion in the proximal tibia of a child is noted, which is characteristic for an osteogenic sarcoma.
Osteosarcoma. An extremely sclerotic lesion in the proximal tibia of a child is noted, which is characteristic for an osteogenic sarcoma.
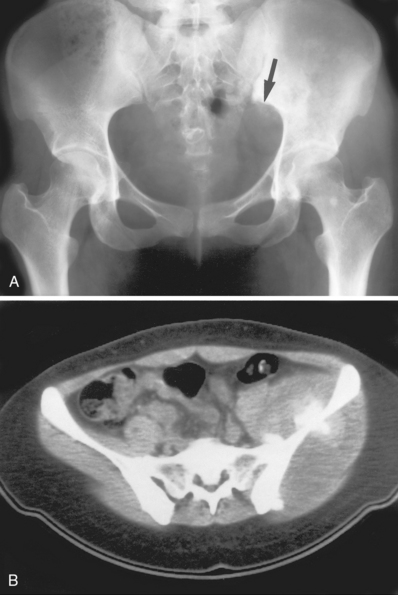
 Osteosarcoma. A, A subtle sclerotic lesion is seen in the left ilium adjacent to the sacroiliac joint that was initially diagnosed as osteitis condensans ilii, a benign entity. Because of persistent pain the patient returned for a follow-up visit, at which time a small amount of cortical destruction on the pelvic brim was noted (arrow). B, A CT scan was obtained and showed a large soft tissue mass and tumor new bone around the ilium, which is characteristic for an osteogenic sarcoma.
Osteosarcoma. A, A subtle sclerotic lesion is seen in the left ilium adjacent to the sacroiliac joint that was initially diagnosed as osteitis condensans ilii, a benign entity. Because of persistent pain the patient returned for a follow-up visit, at which time a small amount of cortical destruction on the pelvic brim was noted (arrow). B, A CT scan was obtained and showed a large soft tissue mass and tumor new bone around the ilium, which is characteristic for an osteogenic sarcoma.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Malignant tumors