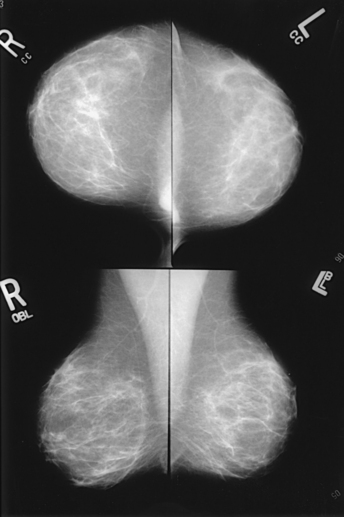
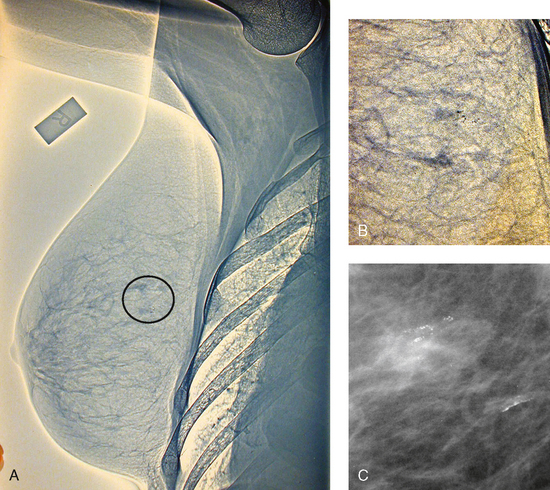
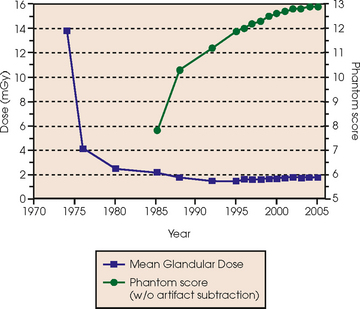
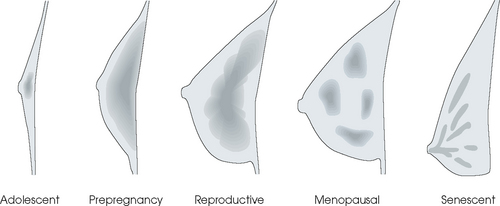
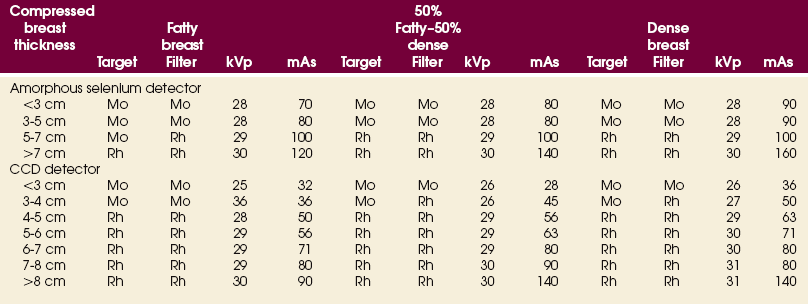
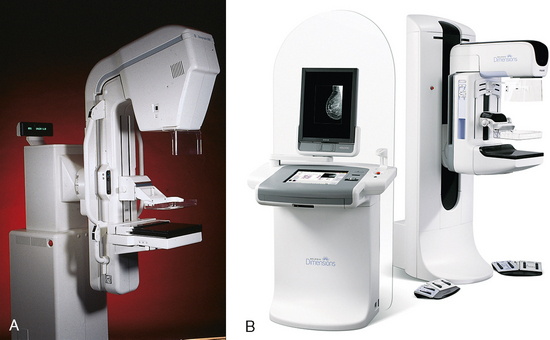
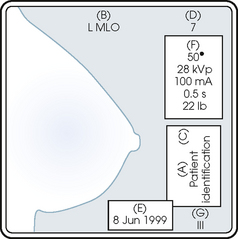
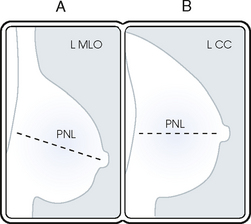
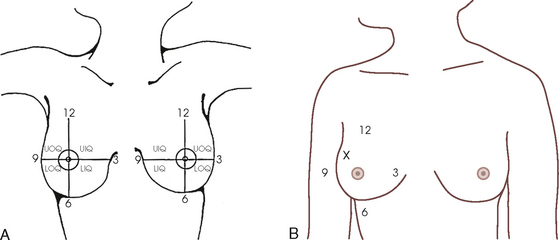
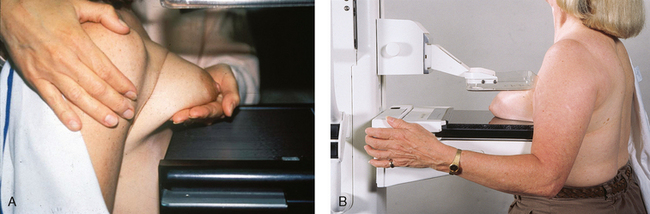
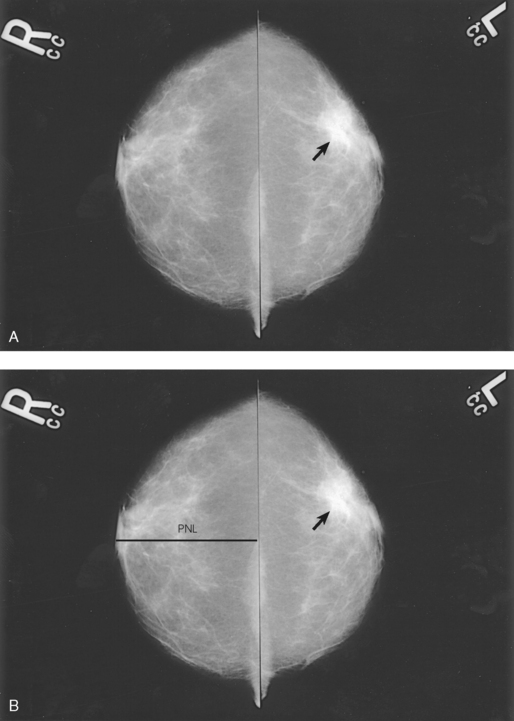
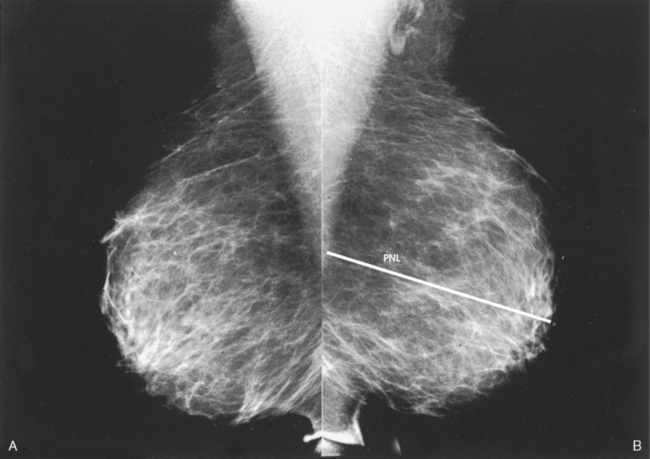
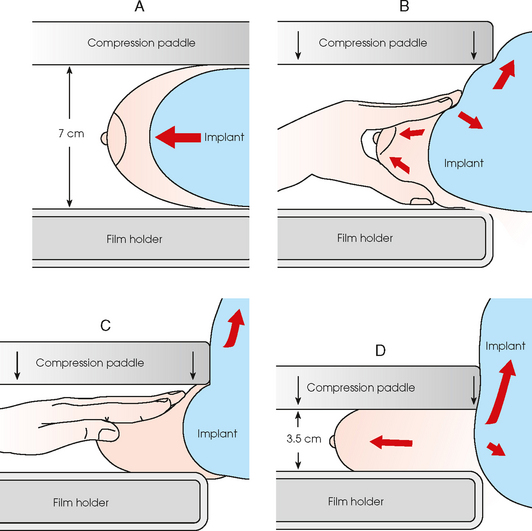
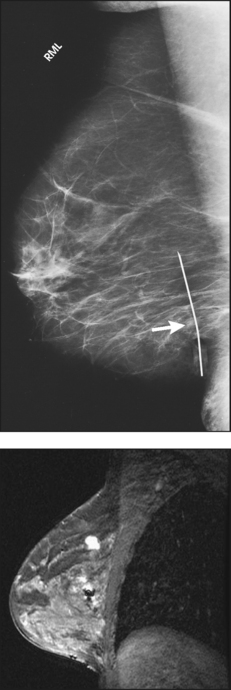
23 Summary of mammography projections Routine projections of the breast Routine projections of the augmented breast Routine projections of the male breast Significant mammographic findings Localization of nonpalpable lesions Ductography (examination of milk ducts) Full-field digital mammography Breast magnetic resonance imaging Thermography and diaphanography INTRODUCTION AND HISTORICAL DEVELOPMENT Despite its frequency, breast cancer is one of the most treatable cancers. Because this malignancy is most treatable when it is detected early, efforts have been directed toward developing breast cancer screening and early detection methods. Breast cancer mortality rates have declined by 2.3% per year from 1990-2000 in all women, with larger increases in women younger than 50 years of age. This decline is most likely the result of earlier detection and improved treatments.1 1. Patients in the early stage of the disease respond well to treatment. 2. Patients with advanced disease do poorly. 3. The earlier the diagnosis, the better the chances of survival. Reflecting these principles, the theory of removing all palpable breast masses in hopes of finding earlier cancers was developed, and it was recognized that careful physical examination of the breast could detect some early breast cancers. Most patients with breast cancer still were not diagnosed until their disease was advanced, however. This fact, coupled with the dismal breast cancer survival statistics, highlighted the need for a tool for the early detection of breast cancer. Mammography filled that need (Fig. 23-1). In 1913, Soloman, a German physician, reported the radiographic appearance of breast cancers. Using radiographic studies of cancerous breasts removed at surgery, he described the mechanism of how breast cancer spread. The first published radiograph of a living person’s breast, made by Kleinschmidt, appeared in a 1927 German medical textbook on malignant tumors. Although publications on mammography appeared in South America, the United States, and Europe during the 1930s, the use of mammography for the diagnosis of breast cancer received little clinical interest. A few pioneers, including LeBorgne in Uruguay, Gershon-Cohen in the United States, and Gros in Germany, published excellent comparisons of mammographic and pathologic anatomy and developed some of the clinical techniques of mammography. At that time, the significance of breast microcalcifications was also well understood. By the mid-1950s, mammography was considered a reliable clinical tool because of such refinements as low-kilovoltage x-ray tubes with molybdenum targets and high-detail, industrial-grade x-ray film. During this time, Egan in the United States and Gros in Germany popularized the use of mammography for diagnosing and evaluating breast cancer. Breast xerography was introduced in the 1960s and was popularized by Wolfe and Ruzicka. Xerography substantially reduced the radiation dose received by the patient compared with the dose received using industrial-grade x-ray film (Fig. 23-2). Because many physicians found xerographic images easier to understand and evaluate, xeromammography became widely used for evaluating breast disease. The first attempts at widespread population screening began at this time. The Breast Cancer Detection Demonstration Project (BCDDP) was implemented in 1973. In this project, 280,000 women underwent annual screening for breast cancer for 5 years at 29 locations throughout the United States. Organized by the American Cancer Society and the National Cancer Institute, this project showed unequivocally that screening, physical examination, mammography, and BSE could provide an early diagnosis. In the BCDDP, more than 41% of all the cancers were found using only mammography, and an even greater proportion of early breast cancers were found only with mammography. The BCDDP was not designed to show that early detection of breast cancer would lead to increased survival rates, but definite evidence from carefully controlled studies in the Netherlands, Sweden, and Germany showed that early diagnosis of breast cancer leads to an increase in curability. In the United States, the Health Insurance Plan study in New York City performed mammography screenings on women older than 50 years and showed the same benefits in reduced mortality rates after early diagnosis of breast cancer. Mean glandular dose provides the best indicator of radiation risk to a patient. In 1997, the average mean glandular dose for a two-projection screen-film-grid mammogram for all facilities in the United States inspected under MQSA was 320 mrad.1 Using that level as a gauge, the lifetime risk of mortality from mammography-induced radiation is 5 deaths per 1 million patients. In other terms, the risk received from having an x-ray mammogram using a screen-film combination is equivalent to smoking several cigarettes, driving 60 miles in an automobile, or being a 60-year-old man for 10 minutes. Fig. 23-3 shows a chart displaying average values for mean glandular dose and estimates of image quality in mammography for the period from the early 1970s to 2005. Doses in mammography have consistently decreased with time, with the most substantial reductions in dose occurring from the early 1970s to the early 1980s. Image quality data are presented from the mid-1980s to the present and show consistent improvement with time.2 A woman whose daughter, sister, or mother previously developed breast cancer, especially at an early age, is at higher risk of developing the disease. Studies have shown, however, that only 13.6% of known breast cancers are found in women with a family history of the disease. A true genetic disorder has been identified in only 5% to 10% of women with breast cancer.1 Although family history is an important risk factor, women with no family history should be aware that they are also at risk. Female breasts vary considerably in size and shape, depending on the amount of fat and glandular tissue and the condition of the suspensory ligaments. Each breast is usually cone-shaped, with the base or posterior surface of the breast overlying the pectoralis major and serratus anterior muscles. These muscles extend from the second or third rib inferiorly to the sixth or seventh rib and from near the lateral margin of the sternum laterally toward the anterior axillary plane. An additional portion of breast tissue, the axillary prolongation or axillary tail (AT), extends from the upper lateral base of the breasts into the axillary fossa (Fig. 23-4). Fig. 23-4 Relationship of breast to chest wall. Note extension of breast tissue posteriorly into axilla. The openings of each acinus join to form lactiferous ductules that drain the lobules, which join to form 15 to 20 lactiferous ducts, one for each lobe. Several lactiferous ducts may combine before emptying directly into the nipple. As a result, there are usually fewer duct openings on the nipple than there are breast ducts and lobes. The individual lobes are incompletely separated from each other by the Cooper ligaments. The space between the lobes also contains fatty tissue and additional connective tissue. A layer of fatty tissue surrounds the gland except in the area immediately under the areola and nipple (Fig. 23-5). Fig. 23-5 A, Sagittal section through female breast, illustrating structural anatomy. B, Breast anterior view. The glandular and connective tissues of the breasts are soft tissue–density structures. The ability to show radiographic detail within the breast depends on the fat within and between the breast lobules and the fat surrounding the breasts. The postpubertal adolescent breast contains primarily dense connective tissue and casts a relatively homogeneous radiographic image with little tissue differentiation (Fig. 23-6). The development of glandular tissue decreases radiographic contrast. During pregnancy, significant hypertrophy of glands and ducts occurs within the breasts. This change causes the breasts to become extremely dense and opaque. After the end of lactation, considerable involution of glandular and parenchymal tissues usually occurs, and these tissues are replaced with increased amounts of fatty tissue. Fat accumulation varies markedly among individuals. This normal fat accumulation significantly increases the natural radiographic contrast within the breasts (Fig. 23-7). The breasts of patients with fibrocystic parenchymal conditions may not undergo this involution (Fig. 23-8). The glandular and connective tissue elements of the breast can regenerate as needed for subsequent pregnancies. After menopause, the glandular and stromal elements undergo gradual atrophy (Fig. 23-9). External factors such as surgical menopause and ingestion of hormones may inhibit this normal process. From puberty through menopause, mammotrophic hormones influence cyclic changes in the breasts. The glandular and connective tissues are in a state of constant change (Fig. 23-10). EVOLUTION OF MAMMOGRAPHY SYSTEMS Diligent research and development began in the 1960s, and the first dedicated mammography unit was introduced in 1967 by CGR (France) (Fig. 23-11). In the 1970s, increased awareness of the elevated radiation doses prevalent in mammography served as the catalyst for the rapid progression of imaging systems. In the 1970s and early 1980s, xeromammography, named for the Xerox Corporation that developed it, was widely used (see Fig. 23-2). This method used much less radiation than the direct-exposure, silver-based films that were available. Eventually, film manufacturers introduced several generations of mammography film-screen systems that used even less exposure and improved tissue visualization. Each subsequent new system showed improvement in contrast and resolution while minimizing patient dose. The high-frequency generators offer more precise control of kilovolt (peak) (kVp), milliamperes (mA), and exposure time. The linearity and reproducibility of the radiographic exposures using high-frequency generators is uniformly excellent. The greatest benefit of these generators may be the efficient waveform output that produces a higher effective energy x-ray beam per set kVp and mA. High-frequency generators are not as bulky, and they can be installed within the single-standing mammography unit operating on single-phase incoming line power, facilitating easy installation and creating a less intimidating appearance (Fig. 23-12). Specialized grids were developed for mammography during the 1980s to reduce scatter radiation and increase the image contrast in mammography. Most units employ moving linear focused grids, but some manufacturers have developed very specialized grids. The Hologic (Lorad) High Transmission Cellular (HTC) Grid employs a honeycomb-pattern, multidirectional design. All dedicated mammography units today, with the exception of slit-scan digital units, still employ grids. Because of the sensitivity of the radiographic films and imaging techniques used for mammography, artifacts are common. It is advisable to dress patients in open-front gowns because the breast must be bared for the examination. Patients should remove any deodorant and powder from the axilla region and breast because these substances can resemble calcifications on the resultant image. Before the breast is radiographed, a complete history is taken, and a careful physical assessment is performed, noting all biopsy scars, palpable masses, suspicious thickenings, skin abnormalities, and nipple alterations (Fig. 23-13). • If possible, examine previous mammographic studies of patients who are undergoing subsequent mammography screening. These images should be evaluated for positioning, compression, and exposure factors to determine whether any improvement in image quality is required for the current study. Position the breast consistently so that any lesion can be accurately localized and a valid comparison can be made with prior studies. • Determine the correct IR size for the patient, and use the smallest possible size to image all of the breast tissue fully. Positioning the breast on a surface that is too large causes the skin and muscles to overextend, reducing the amount of posterior tissue imaged. • Explain the procedure simply and completely to the patient before beginning the examination. It should never be assumed that the patient is fully aware of what the mammographer is about to do, even if the patient has had prior examinations. • In many cases, the routine projections do not sufficiently show all of the breast tissue, and additional projections may be necessary. To allay patient concerns, the mammographer should explain to the patient before beginning the procedure why additional projections are sometimes needed and that they do not indicate a potential problem. • Before positioning the patient’s breast and applying compression, consider the natural mobility of the breast so that patient discomfort can be minimized. The inferior and lateral portions of the breast are mobile, whereas the superior and medial portions are fixed. Whenever possible, the mobile tissues should be moved toward the fixed tissues. • For each of the two basic breast projections, ensure that the breast is firmly supported and adjusted so that the nipple is directed forward. • Profile the nipple, if possible. Obtaining an image of the posterior breast tissue should be the primary consideration, and positioning of the nipple in profile is not always possible. An additional projection to profile the nipple can be obtained if necessary. Alternatively, a marker may be used to locate clearly the nipple that is not in profile, in which case an additional image may not be needed. • Apply proper compression to the breast. Compression is an important factor in achieving a high-quality mammogram. The primary objective of compression is to produce uniform breast thickness from the nipple to the posteriormost aspect of the breast. Properly applied compression spreads the breast so that the tissue thickness is more evenly distributed over the image and better separation of the glandular elements is achieved. A rigid, radiolucent mammography compression paddle facilitates breast compression. Generally, compression is applied initially using a hands-free control and then applied manually during the final phase of compression. The compression should be taut but not painful. The skin of a properly compressed breast should feel tight when lightly tapped with the fingertips. When evaluating images, compare the degree of compression with previous mammograms and note any variations. If a patient is unable to tolerate an adequate amount of compression, document this information on the patient history form for the radiologist. • Place identification markers (Fig. 23-14) according to the following standard convention: A Before processing, photographically expose a permanent identification label that includes the facility’s name and address; the date of the examination; and the patient’s name, age, date of birth, and medical number on the image. Include the initials of the person performing the examination on the identification label (C). B On the IR near the patient’s axilla, place a radiopaque marker indicating the side examined and the projection used (Table 23-1). TABLE 23-1 Labeling codes for mammographic positioning From Bassett L et al, editors: Quality determinants of mammography, AHCPR Pub No 95-0632, Rockville, MD, 1994, U.S. Department of Health and Human Services. C Label the mammography cassette with an identification number (Arabic numeral is suggested by the ACR). • Mammography film labeling may also include the following: D A separate date sticker or perforation E A label indicating the technical factors used: kVp, milliampere-seconds (mAs), target material, degree of obliquity, density setting, exposure time, and compression thickness. This is often included on the automatic identification labeling system that most manufacturers now offer with their units. F Facilities with more than one unit must identify the mammographic unit used (Roman numerals are suggested by the ACR). G For FFDM images, all of the aforementioned pertinent information should be included in the DICOM header. The information should also be seen on the processed image or, if possible, used in a DICOM overlay that can be turned on or off as needed by the radiologist, to prevent interference while interpreting the image. • For patients with palpable masses, a radiopaque (BB or X-spot) marker may be used to identify the location of the mass. A different type of radiopaque marker may be used to identify skin lesions, scars, or moles. This is determined by the policy of the facility. • When using automatic exposure control (AEC), position the variable-position detector at the chest wall, the mid-breast, or the anterior breast, depending on breast composition and size. The appropriate location of the AEC detector must be determined for each individual patient. If possible, the detector should be placed under the most glandular portion of the breast, usually just posterior to the nipple. • When reviewing images, assess contrast and density for optimal differentiation of breast tissues. Anatomic markers should be visible. The projections of one breast should be compared with the same projections of the contralateral breast to evaluate symmetry and consistency of positioning. All images should be absent of motion blur, artifacts, and skin folds. Images must be evaluated for potentially suspicious lesions and calcifications that may require image enhancement methods. • To evaluate whether sufficient breast tissue is shown, the radiographer should measure the depth of the breast from the nipple to the chest wall on the CC and MLO projections. The posterior nipple line (PNL) is an imaginary line that is “drawn” obliquely from the nipple to the pectoralis muscle or edge of the image, whichever comes first on the MLO projection. On the CC projection, the PNL is “drawn” from the nipple to the chest wall or to the edge of the image, whichever comes first. The PNL on the CC should be within ⅓ inch (1 cm) of depth of the PNL on the MLO projection (Fig. 23-15). • Between examinations, use a disinfectant to clean the image receptor tray surface, compression paddle, patient handle grips, and face guard. • If practical, a heating pad or commercially available mammography image receptor cover may be used to warm the image receptor tray surface and to enhance patient comfort. • Mammography is a team effort involving the patient and the mammographer. Acknowledge the individual needs of each patient to facilitate the cooperation and trust necessary to complete the procedure successfully. The nature of the interaction between the radiographer and the patient is likely to determine whether the patient chooses to have subsequent mammograms. Descriptive terminology has been developed for the referring physician, the technologist, and the radiologist all to communicate efficiently regarding an area of concern within a breast. When describing an area of concern, the laterality (right or left) must accompany the description (Fig. 23-16). Mammography is routinely performed using the CC and MLO projections. • While standing on the medial side of the breast to be imaged, elevate the inframammary fold to its maximal height. • Adjust the height of the C-arm to the level of the inferior surface of the patient’s breast. • Use both hands to pull the breast gently onto the image receptor holder, while instructing the patient to press the thorax against the image receptor. Have the patient lean slightly forward from the waist. • Keep the breast perpendicular to the chest wall. The technologist should use his or her fingertips to pull the posterior tissue gently forward onto the IR. • Center the breast over the AEC detector, with the nipple in profile if possible. • Immobilize the breast with one hand, being careful not to remove this hand until compression begins. • Use the other hand to drape the opposite breast over the corner of the image receptor. This maneuver improves demonstration of the medial tissue. • Have the patient hold onto the grab bar with the contralateral hand; this helps steady the patient as you continue positioning. • Placing your arm against the patient’s back with your hand on the shoulder of the affected side, make certain the patient’s shoulder is relaxed and in external rotation. • Rotate the patient’s head away from the affected side. • Lean the patient toward the machine, and rest the patient’s head against the face guard. • Make certain no other objects obstruct the path of the beam. • With the hand on the patient’s shoulder, gently slide the skin up over the clavicle. • Using the hand that is anchoring the patient’s breast, pull the lateral tissue on the image receptor without sacrificing medial tissue. • Inform the patient that compression of the breast will be used. Bring the compression paddle into contact with the breast while sliding the hand toward the nipple. • Slowly apply compression until the breast feels taut. • Check the medial and lateral aspects of the breast for adequate compression. • Instruct the patient to indicate whether the compression becomes uncomfortable. • After full compression is achieved and checked, move the AEC detector to the appropriate position, and instruct the patient to stop breathing (Fig. 23-17). • Determine the degree of obliquity of the C-arm apparatus by rotating the tube until the long edge of the image receptor is parallel to the upper third of the pectoral muscle of the affected side. The degree of obliquity should be between 30 degrees and 60 degrees, depending on the patient’s body habitus. • Adjust the height of the C-arm so that the superior border is level with the axilla. • Instruct the patient to elevate the arm of the affected side over the corner of the image receptor and to rest the hand on the adjacent handgrip. The patient’s elbow should be flexed and resting posterior to the image receptor. • Place the upper corner of the image receptor as high as possible into the patient’s axilla between the pectoral and latissimus dorsi muscles so that the image receptor is behind the pectoral fold. • Ensure that the patient’s affected shoulder is relaxed and leaning slightly anterior. Placing the flat surface of the hand along the lateral aspect of the breast, gently pull the patient’s breast and pectoral muscle anteriorly and medially. • Holding the breast between the thumb and fingers, gently lift it up, out, and away from the chest wall. • Rotate the patient’s body toward the image receptor while asking the patient to bend slightly at the waist. • Center the breast with the nipple in profile if possible, and hold the breast in position. • Hold the breast up and out by rotating the hand so that the base of the thumb and the heel of the hand support the breast (fingers are pointing away from breast). • Inform the patient that compression of the breast will be used. Continue to hold the breast up and out while sliding the hand toward the nipple as the compression paddle is brought into contact with the breast. • Slowly apply compression until the breast feels taut. The corner of the compression paddle should be inferior to the clavicle. • Check the superior and inferior aspects of the breast for adequate compression. • Instruct the patient to indicate whether the compression becomes uncomfortable. • Pull down on the patient’s abdominal tissue to open the inframammary fold. • Instruct the patient to hold the opposite breast away from the path of the beam. • After full compression is achieved, move the AEC detector to the appropriate position, and instruct the patient to stop breathing (Fig. 23-19). • Perpendicular to the base of the breast • The C-arm apparatus is positioned at an angle determined by the slope of the patient’s pectoral muscle (30 to 60 degrees). The actual angle is determined by the patient’s body habitus: Tall, thin patients require steep angulation, whereas short, stout patients require shallow angulation. The initial two projections may be combined with the Eklund, or implant displaced (ID), technique. For the Eklund method, the implant is pushed posteriorly against the chest wall so that it is excluded from the image, and the breast tissue surrounding the implant is pulled anteriorly and compressed. This positioning improves compression of breast tissue and visualization of breast structures. The CC and MLO projections are often performed using the ID technique. MRI is currently the most commonly used modality for diagnostic evaluation of augmented breasts. Although MRI offers several diagnostic advantages, the cost and time-consuming nature of the procedure inhibits its use as a screening modality for patients who have undergone augmentation. It may be used as a screening tool for women who have undergone reconstruction after breast cancer surgery. MRI has proved useful as a preoperative tool in locating the position of an implant, identifying the contour of the deformity, and confirming rupture and leakage migration patterns. The sensitivity and specificity of MRI have been 94% and 97%.1 • Turn the AEC off, and preselect a manual technique. • Follow the same positioning sequence as for the standard CC projection. • Inform the patient that compression of the breast will be used. Bring the compression paddle into contact with the breast, and slowly apply enough compression to immobilize the breast only. Compression should be minimal. The anterior breast tissue should still feel soft. • Select the appropriate exposure factors, and instruct the patient to stop breathing. Structures shown: The image should show the entire implant and surrounding posterior breast tissue with suboptimal compression of the anterior fibroglandular breast tissue (Fig. 23-21). Fig. 23-21 Bilateral, four-image CC and MLO examination of augmented breasts of a 37-year-old woman. Implants have been surgically placed behind pectoral muscle. Additional radiographs should be obtained using Eklund (ID) technique to complete the eight-radiograph study (see Fig. 23-22). CRANIOCAUDAL PROJECTION WITH IMPLANT DISPLACED (CC ID) • While standing on the medial side of the breast to be imaged, elevate the inframammary fold to its maximal height. • Adjust the height of the C-arm to the level of the inferior surface of the breast. • Standing behind the patient, place both arms around the patient and locate the anterior border of the implant by walking the fingers back from the nipple toward the chest wall. • When the anterior border of the implant has been located, gently pull the anterior breast tissue forward onto the image receptor (Fig. 23-22). Use the hands and the edge of the image receptor to keep the implant displaced posteriorly. • Center the breast over the AEC detector with the nipple in profile if possible. • Hold the implant back against the chest wall. Slowly apply compression to the anterior skin surface, being careful not to allow the implant to slip under the compression paddle. As compression continues, the implant should be seen bulging behind the compression paddle. • Apply compression until the anterior breast tissue is taut. Compared with the full-implant projection, an additional ¾ to 2 inches (2 to 5 cm) of compression should be achieved with the implant displaced. • Instruct the patient to indicate whether the compression becomes uncomfortable. • When full compression is achieved, move the AEC detector to the appropriate position and instruct the patient to stop breathing. Structures shown: This projection shows the implant displaced posteriorly. The anterior and central breast tissue is seen projected free of superimposition with uniform compression and improved tissue differentiation (Fig. 23-23). Fig. 23-23 Bilateral, four-image with ID examination of the same patient as in Fig. 23-20, using Eklund (ID) technique. Implants are pushed back for better visualization of surrounding breast tissue.
MAMMOGRAPHY
Principles of Mammography
RISK VERSUS BENEFIT
RISK FACTORS
Family history
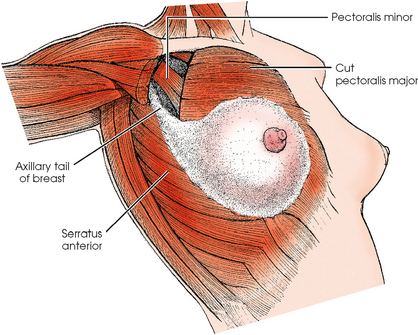
Breast
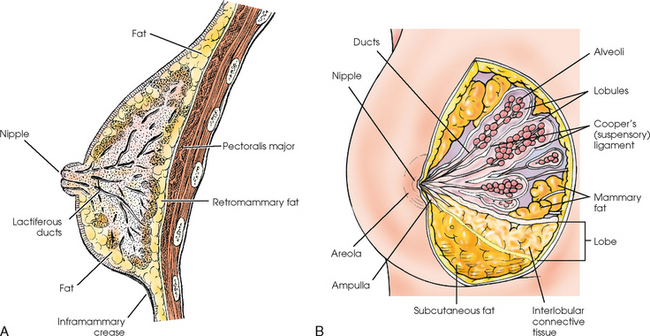
Tissue Variations
SUMMARY OF PATHOLOGY
Condition
Definition
Breast carcinoma
Malignant new growth composed of epithelial cells
Calcification
Deposit of calcium salt in tissue; characteristics may suggest either benign or malignant processes
Cyst
Closed epithelial sac containing fluid or a semisolid substance
Epithelial hyperplasia
Proliferation of the epithelium of the breast
Fibrosis
Formation of fibrous tissue in the breast
Tumor
New tissue growth where cell proliferation is uncontrolled
Fibroadenoma
Benign tumor of breast containing fibrous elements
Intraductal papilloma
Benign, neoplastic papillary growth in a duct
Breast Imaging
MAMMOGRAPHY EQUIPMENT
METHOD OF EXAMINATION
EXAMINATION PROCEDURES
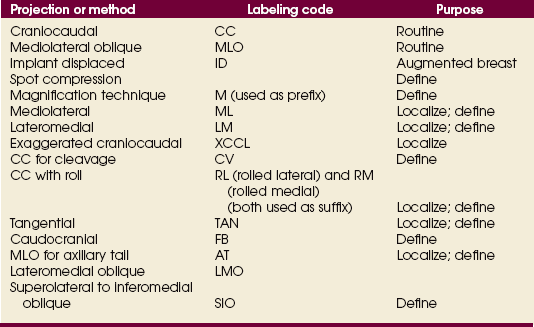
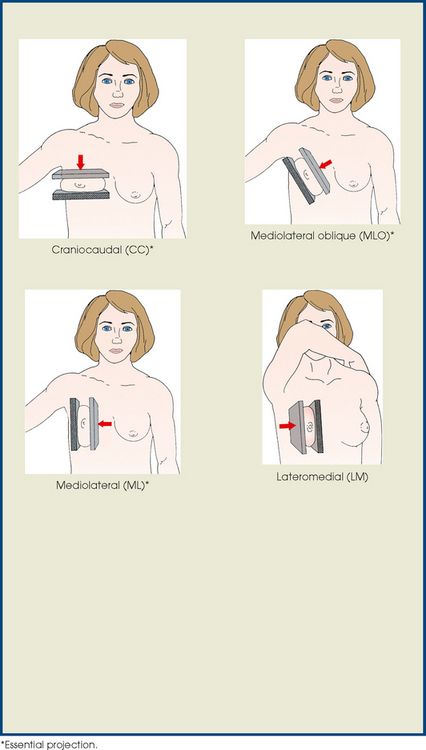
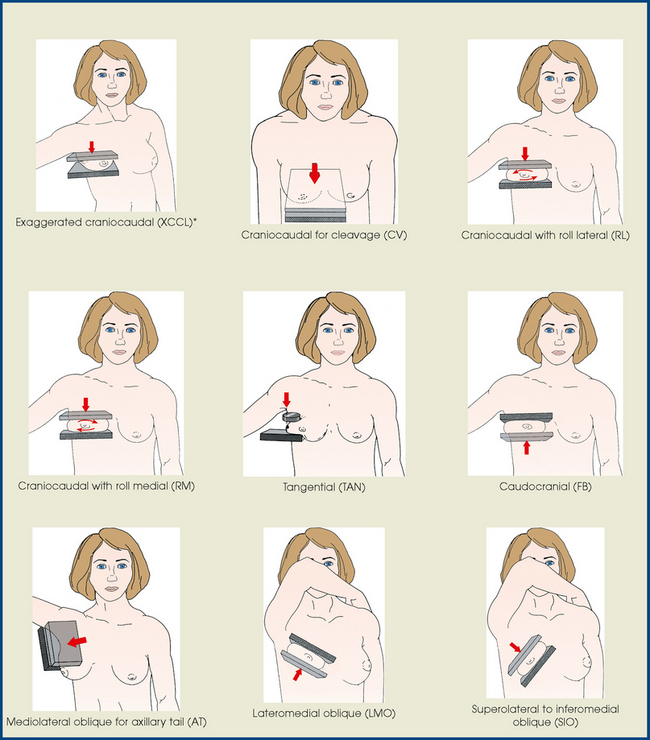
Summary of Mammography Projections
DESCRIPTIVE TERMINOLOGY
Routine Projections of the Breast
![]() CRANIOCAUDAL (CC) PROJECTION
CRANIOCAUDAL (CC) PROJECTION
![]() MEDIOLATERAL OBLIQUE (MLO) PROJECTION
MEDIOLATERAL OBLIQUE (MLO) PROJECTION
Routine Projections of the Augmented Breast
CRANIOCAUDAL (CC) PROJECTION WITH FULL IMPLANT
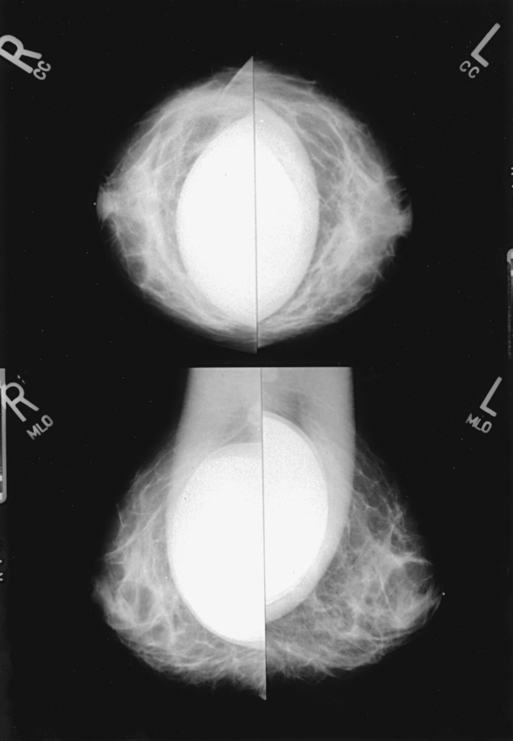
Augmented Breast
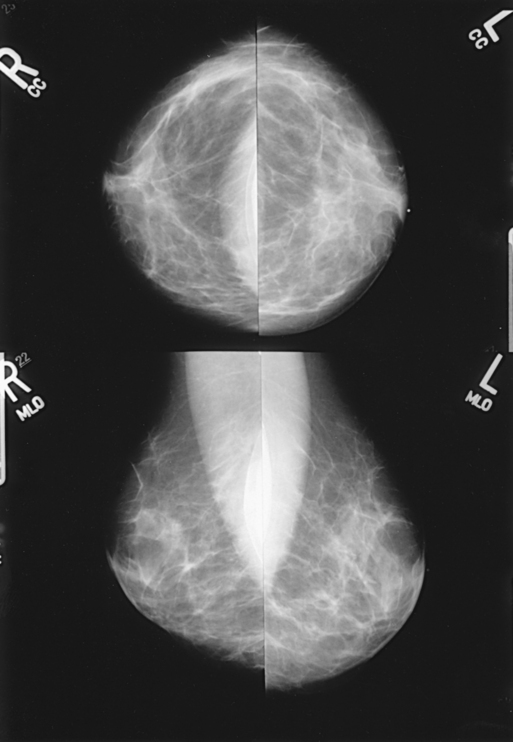
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree