Vascular anomalies are often poorly managed for a number of reasons: they are uncommon (other than the true infantile hemangioma), their mode of presentation is extremely variable, their classification has been very confusing and is still poorly understood by the majority of doctors, and their treatment is challenging. The interested interventional radiologist is in an ideal position to play a major, if not the lead, role in patient management. So many aspects of assessment and treatment require radiologic input, and it is essential that this involvement start at the time of referral and the patient’s first visit to the outpatient clinic. It is not good practice for a radiologist to accept a referral for endovascular treatment of a vascular malformation without first assessing the patient.
For a clinician familiar with these anomalies, the diagnosis is almost always straightforward. A thorough patient history and physical examination usually make it clear which patients require, and are likely to benefit from, treatment. Patients and referrers are invariably keen for definitive intervention to cure what are often highly disfiguring or lifestyle-limiting conditions, but most vascular malformations cannot be cured, and the aim of any intervention is usually long-term symptom control. The radiologist’s role is as much that of gatekeeper as interventionalist, stressing the current limitations of medical science, advising against heroic and often fruitless major intervention, and helping patients come to terms with their condition. A holistic approach is key, and the care of a patient with a vascular anomaly is ideally undertaken by a multidisciplinary team.
Classification
In 1982 Mulliken and Glowacki proposed what was at that time a very new way of classifying vascular anomalies. Their system has been modified very little since then, with only minor changes made by the International Society for the Study of Vascular Anomalies when it was established in 1992. This is now the most widely recognized system in use, encouraging management based on underlying histology and natural history of the lesion subtypes. The dichotomous Mulliken and Glowacki classification separates vascular tumors from vascular malformations. The vascular tumor arm includes the common infantile hemangiomas as well as rarer congenital hemangiomas and other vascular tumors, many of which are high flow. The vascular malformation arm comprises low-flow venous and lymphatic malformations and high-flow arteriovenous malformations (AVMs) ( Table 18.1 ).
| Type | Characteristics |
|---|---|
| Infantile hemangioma |
|
| Congenital hemangioma | Present at birth |
| Noninvoluting |
|
| Rapidly involuting |
|
| Other Types | |
| Tufted angioma | |
| Kaposiform hemangioendothelioma | |
| Pyogenic granuloma | |
Vascular Tumors
Most vascular tumors are infantile hemangiomas, which occur in up to 12% of Caucasian infants by the age of 1 year. Congenital hemangiomas share some of the characteristics of the common infantile lesions but have different natural histories. Other tumors in this arm of the classification system are rare but are more likely to involve interventional radiology. These include tufted angiomas and kaposiform hemangioendotheliomas.
Infantile Hemangioma
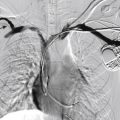
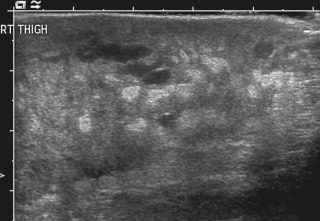
Infantile hemangiomas are by far the most common lesions in this subgroup. They are not present at birth, appearing at around 2 to 4 months of age. Typically they demonstrate a highly active period of growth and proliferation over several months and then slowly involute, disappearing during school age. It is this natural history that clearly separates them from vascular malformations that, in contrast, are present at birth and grow commensurately with the child. Infantile hemangiomas are usually superficial lesions with a characteristic bright red color and are easily diagnosed clinically, rarely troubling the radiologist. Deeper seated lesions will not exhibit skin staining. Such rapidly growing masses generate consternation and urgent referral. The diagnosis is simple because the imaging features are usually characteristic. Ultrasound demonstrates a well-defined echogenic lesion that is highly vascular with large central feeding arteries and draining veins ( Fig. 18.1 ). The highly arterialized nature of the lesion simply reflects its extremely active proliferation. Occasionally, biopsy may be required to allay concerns. Infantile hemangiomas express glucose transporter type 1 (GLUT1), a simple diagnostic marker. Core needle biopsy, when necessary to exclude more serious pathology, is usually straightforward under ultrasound guidance because lesions are typically superficial. A 16G coaxial technique is advocated in view of the highly vascular nature of these lesions, allowing a single pass through the tumor capsule and plugging of the biopsy track with Gelfoam pellets (Pfizer, New York, NY); complications, in the authors’ experience, are rare.
Perhaps surprisingly most of these highly vascular tumors do not cause vascular steal or cardiac compromise and can be managed conservatively. Intervention is indicated when lesions cause significant mass effect or disfigurement. Subglottic lesions and those that obstruct the visual axis are of particular concern. Pharmacologic management is now the mainstay of treatment in this group, with β-blockers the preferred first-line therapy. β-Blockers induce rapid shrinkage of infantile hemangiomas over a matter of weeks if administered in the proliferative phase. Although drug administration needs to be carefully managed, this provides a simple and noninvasive method of disease control and means that embolization is now almost never required for large, hypervascular lesions causing systemic effects.
Congenital Hemangioma
This hemangioma subgroup merits brief mention because of significant differences in their natural history and prognosis when compared with infantile hemangiomas, which influences management decisions. Congenital hemangiomas are fully formed at birth and this feature alone distinguishes them from the infantile group. They are similar in appearance but have subtle differences in color and contour compared with the classic infantile lesions. Imaging features are not strikingly different from those of infantile hemangiomas, although the lesions are often more heterogeneous, and calcification can be seen.
Congenital hemangiomas form two distinct clinical subgroups defined by their natural history. Some involute very rapidly in the first few months of life and are termed rapidly involuting congenital hemangioma (RICH), and others never involute ( noninvoluting congenital hemangioma [NICH]). Both types are histologically and immunophenotypically distinct from infantile hemangiomas. Neither expresses GLUT1. Biopsy of such lesions is sometimes helpful to allow clinicians to predict outcome. NICHs are usually small and clinically inconsequential, RICHs can be extremely large and hypervascular at birth, causing cardiovascular instability and coagulopathy. Urgent embolization of very large congenital hemangiomas is often requested but should be avoided if possible, because these lesions will involute quickly without intervention.
Other Vascular Tumors
Infantile and congenital hemangiomas make up the vast majority of lesions in the vascular tumor arm of the Mulliken and Glowacki classification of vascular anomalies, but it is important to recognize that other much less common lesions exist. These include tufted angioma, kaposiform hemangioendothelioma (KHE), hemangiopericytoma, and pyogenic granuloma. Recognition of tufted angiomas and KHEs in particular is important for two reasons: they often have a more unusual aggressive appearance despite their benign histology ( Fig. 18.2 ), precipitating direct referral to oncologists, and they are sometimes associated with the Kasabach-Merritt phenomenon (KMP). Both are GLUT1-negative on biopsy and have characteristic histology, so biopsy is often useful. Kasabach-Merritt phenomenon describes a pattern of variable but often severe coagulopathy and thrombocytopenia; it is seen in association with a variety of soft tissue lesions, but most commonly KHE. Children with vascular tumors who exhibit KMP show a variable response to therapy, and morbidity and mortality rates are high. Evolving pharmacologic approaches to other vascular tumors may have a role to play in the management of these lesions. Embolization remains an alternative approach in an attempt to reduce tissue bulk and downregulate the drivers for KMP, but evidence is lacking.
Liver Vascular Tumors in Childhood
Vascular tumors of the liver deserve special mention because of the large volume of inaccurate and misleading literature about them in publication and confusion over their management. Lesions in the adult liver, spleen, and bone that have traditionally been described as hemangiomas almost certainly represent small venous malformations and are entities entirely distinct from true liver hemangiomas of infancy.
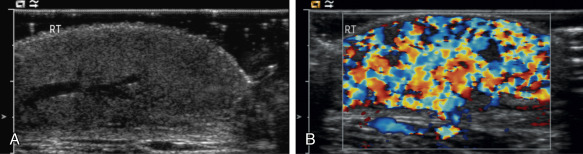
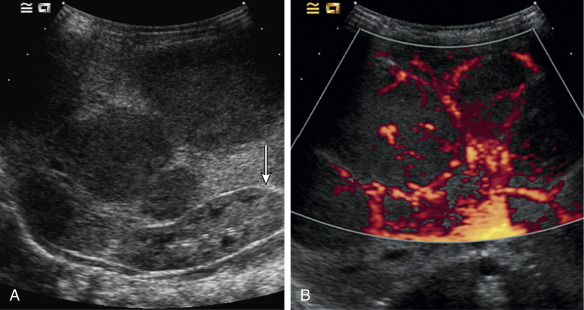
Both infantile and congenital hemangiomas occur in the liver in a proportion of children. The infantile lesions are almost always multifocal or diffuse and associated with multiple cutaneous hemangiomas, whereas the congenital lesions are typically solitary. Ultrasound confirms their high-flow nature and can demonstrate microvascular shunting at an intralesional level; this simply reflects disorganized neovascularity within these rapidly proliferating lesions ( Fig. 18.3 ). It is important that these not be labeled as AVMs because this inevitably leads to high levels of clinical anxiety and the potential for unnecessary intervention. Infantile hemangiomas of the liver have the same natural history as the more common skin lesions, showing slow involution over time; they are very rarely of any clinical significance and can be controlled with β-blockade. Hepatic congenital hemangiomas are far more commonly the RICH subtype rather than NICH, and involute spontaneously and quickly. As elsewhere, biopsy is diagnostic and should guide management. Additionally, serum α-fetoprotein should be measured to exclude hepatoblastoma. Because serum α-fetoprotein concentrations vary widely between individuals in the neonatal period and can be elevated in children with liver hemangiomas, serial measurements are recommended to ensure levels fall rapidly in the first few weeks after birth.
Extensive RICH involvement of the liver, albeit temporary, can cause dramatic hepatomegaly in the neonate, leading to respiratory and cardiac compromise. Thrombocytopenia and coagulopathy can also be seen. In such instances, medical management is key to controlling cardiac and hematologic sequelae. In refractory or fulminant cases, embolization may buy the child some time by reducing lesion size and vascularity until natural involution occurs. A combination of coil and particle embolization is usually successful. Left axillary arterial access is often a useful approach in the neonate, and carbon dioxide angiography minimizes the volume of fluid and iodinated contrast media required in these hemodynamically unstable children.
Vascular Malformations
Low-flow malformations are discussed in detail in Chapter 17 . This chapter focuses on high-flow (arteriovenous) malformations. All vascular malformations are considered to be present at birth, although they may not become apparent until childhood or even adult life. An increase in size during childhood and especially around puberty is common, and many individuals present at this time because of the increasing prominence of the malformation, which may be associated with new or worsening symptoms, including pain, ulceration, and hemorrhage. Progression of high-flow malformations may also occur in response to pregnancy and is occasionally seen after trauma, which may be accidental or iatrogenic—for example, proximal surgical ligation or embolization of feeding arteries and subtotal resection.
On clinical examination, a pulsatile soft tissue swelling is usually evident, with prominence of draining superficial veins. The high-flow nature of a malformation is often clear on simple palpation, but a deeper-seated lesion may be less obviously pulsatile. In such cases, detection of an arteriovenous signal (“machinery murmur”) using a simple handheld Doppler probe in the outpatient clinic will avoid misdiagnosing a high-flow lesion as one that is low-flow; this is clearly an important distinction to make because subsequent imaging and treatment of these two entities is very different. The differential diagnosis of a vascular soft tissue tumor should be considered, although a history of a long-standing preexisting swelling or the clinical finding of associated overlying skin markings will help to confirm the congenital nature of the lesion. Biopsy may be necessary in some individuals if doubt exists as to its true nature. Note should be made of any complications of the malformation, such as thinning of the overlying skin, frank ulceration, infection, and bleeding.
An acquired posttraumatic arteriovenous fistula may have an identical appearance on clinical examination to that of a high-flow AVM. The diagnosis is usually obvious because of a history of antecedent trauma, but some patients will not volunteer this information and indeed may have forgotten a previous injury. This is not uncommon with scalp lesions where a relatively minor injury, which may or may not have been associated with a cutaneous laceration, can rarely present many years later with a pulsatile soft tissue swelling due to an enlarging arteriovenous communication. The demonstration of a single fistulous communication at subsequent angiography will sometimes be the first indication of this diagnosis, which is an important one to make because a cure is likely if the condition is treated appropriately.
Imaging
Other than Doppler ultrasound—which, as mentioned earlier, may be very helpful during initial clinical assessment of a vascular malformation—other imaging of high-flow malformations, although often performed, is rarely essential. The reason is that the decision regarding whether a high-flow malformation requires treatment is based upon clinical findings and symptoms. Many patients require no more than reassurance about the diagnosis and a frank discussion about its nature and natural history. If treatment is felt to be necessary, magnetic resonance imaging (MRI) is the noninvasive modality of first choice to demonstrate the extent of the AVM. Magnetic resonance angiography may be helpful to document its angiographic anatomy, but this is often better visualized by catheter angiography. Bone involvement is best demonstrated on computed tomography (CT). Contrast-enhanced CT is rarely helpful and should not be performed unless there is a contraindication to MRI. Even then it is unlikely to give any more information than catheter angiography, which will be necessary if the decision has been made that treatment is required.
Management
Not all AVMs require treatment. Those that are quiescent are not associated with significant symptoms and cause little in the way of cosmetic deformity are often best left alone. Individuals who do not require treatment should be informed that they should request reassessment if the malformation becomes more troublesome in later years.
Patients with high-flow malformations requiring therapy should undergo formal catheter angiography and in many instances this will be combined with embolization. Some of these lesions will be amenable to surgical excision, usually after embolization, but in many individuals, embolization will provide the best form of treatment.
Embolization
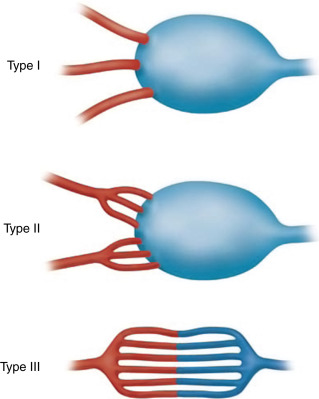
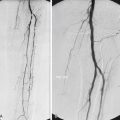
The most important aspect of embolization of a high-flow malformation is an understanding of the anatomy of the vascular connections within it because these have a bearing upon both the method of vascular occlusion and the final result. Houdart et al. classified intracranial AVMs into three main types based upon the anatomy of the arteriovenous communications ( Fig. 18.4 ). This classification is also useful in understanding peripheral AVMs. The types described are:
- •
Type I: arteriovenous. The first identifiable venous component of these AVMs is supplied by three or fewer arterial pedicles.
- •
Type II: arteriolovenous. Here the first identifiable venous component is supplied by more than three (often very many) arterial pedicles.
- •
Type III: arteriolovenulous. Here the arteriovenous communications are so small and numerous they cannot be separately identified, and the first obviously venous component of the lesion is often at some distance from them.