Type of stenosis
Pathology
Intraluminal
Inflammatory: Wegener’s granulomatosis (systemic)
Sarcoidosis
Relapsing polychondritis (with malacia)
Infections: endobronchial tuberculosis
Aspergillosis
Papillomatosis
Hamartoma
Hemangioma
Tracheopathia osteoplastica
Amyloidosis
Pseudotumor
Granulation tissue/granuloma
Broncholith
Foreign body
Mucous plug
Blood clot
Extrinsic compression
Lymphadenopathy
Goiter
Mediastinal cyst
Distortion
Vascular sling
Post pneumonectomy syndrome
Scar/stricture
Post-intubation/tracheotomy stenosis
Idiopathic subglottic/tracheal stenosis
Fibrotic phase of infectious and inflammatory lesion
Anastomotic complications from tracheal surgery and lung transplant
Granulation tissue
Fibrosis
Bronchomalacia
Necrosis/mucosal sloughing Infection
Dynamic airway obstruction
Tracheomalacia
Bronchomalacia
More than half of all the procedures in therapeutic bronchoscopy involve patients with benign airway disease, and nearly 40% are performed on an urgent or emergency basis. A third of all patients with benign pathology undergoing rigid endoscopy have an ECOG functional performance status score of >2. This need for urgent intervention in patients with impaired functional capacity, coupled with the necessity for subspecialized surgical expertise and the recent advancement of bronchoscopic techniques, has challenged the paradigm that nonmalignant airway obstruction should be managed primarily by surgeons. Furthermore, endoscopic treatments on benign disease carry a lower risk of short-term complications, as well as less intraoperative hypoxia and bleeding than similar procedures on patients with malignant pathology. The relationship between therapeutic bronchoscopy and tracheal surgery is in practice intertwined and complimentary. Outcome data are difficult to compare because surgical series often exclude inoperable patients while endoscopic studies focus on only such inoperable cases. There is also no single definitive treatment for any particular pathology or any absolute indication for any particular intervention. Despite the lack of consensus on the choice of definitive therapy, the management principles remain fairly straightforward.
The principles of treating nonmalignant airway obstruction are (1) stabilization of the patient, (2) thorough evaluation within a multidisciplinary context, and (3) careful selection of an appropriate intervention for each case (Fig. 26.1). Endoscopic therapy is often used to secure the airways, and bronchoscopic evaluation is pivotal in the selection of definitive treatment. Bronchoscopy also plays a big role in planning and preparing a patient for surgery. Furthermore, patients who may require specific systemic therapy for infections, connective tissue diseases, or inflammations can be identified. The choice of appropriate therapeutic intervention is determined by the unique characteristics and the benefits/risks of treatment modalities (Fig. 26.1). Disease parameters that include etiology, pathophysiology, natural history, and complicating factors such as malacia or laryngeal involvement also determine treatment outcomes.
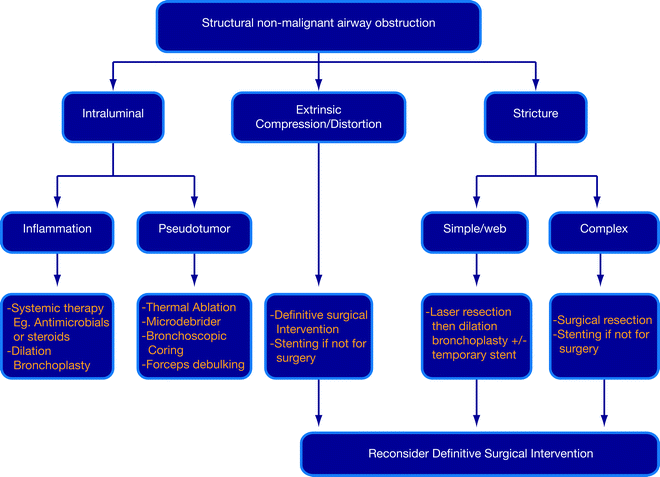

Fig. 26.1
Algorithm for the management of nonmalignant airway obstruction
Stabilization/Immediate Management
In respiratory distress, the airway must first be secured (Fig. 26.2). This is a basic principle of providing any emergency life support. No attempt should be made to push the endotracheal tube forcibly through a tracheal stricture. If a small endotracheal tube cannot traverse the lesion, the tube should be placed just above the lesion and the airway suctioned adequately. Alternatively, a laryngeal mask or a tracheotomy may be needed. Rigid bronchoscopy can then be undertaken to evaluate the lesion and establish a more stable airway. Patients in respiratory distress may not be able to lie supine and often need to be managed in the upright position until anesthetic induction. Heliox which is blended helium (60–80%) and oxygen can be used as a bridge prior to securing the airway. Helium, which is less dense than nitrogen, provides more laminar flow past obstructions and reduces turbulence, as well as the work of breathing. Intravenous induction is preferred over inhalational induction because it is more rapid and does not irritate the airway. Neuromuscular blockade that may precipitate a loss of muscle tone and result in airway collapse especially in the presence of an anterior mediastinal mass is relatively contraindicated except in experienced hands. Intravenous dexamethasone and nebulized L-epinephrine have only been studied in the context of laryngeal edema and cannot be recommended for routine use because of inconclusive results.
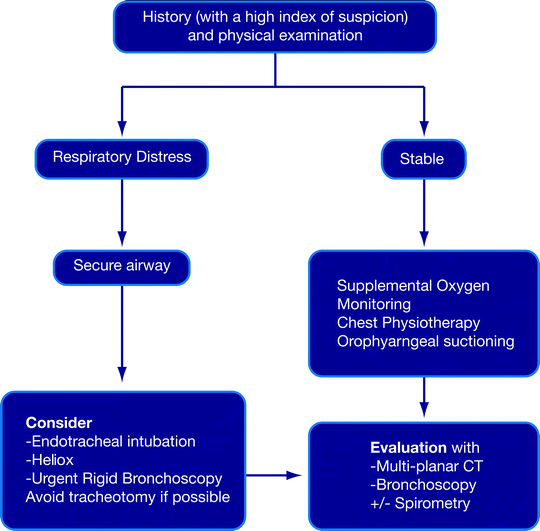

Fig. 26.2
Algorithm for the stabilization and evaluation of nonmalignant airway obstruction
Endotracheal intubation with rigid bronchoscopy not only secures the airway but also permits therapeutic interventions that can palliate the obstruction. The rigid barrel can be used for coring out intraluminal lesions or to sequentially dilate strictures. There is control of bleeding by direct tamponade, and other modalities such as mechanical debridement, lasers, or stents may be employed. In all these interventions, the anesthetist must always have priority in ventilating the patient, and, when requested, endoscopic therapy should temporarily cease and the patient ventilated.
Placement of a tracheotomy as a temporizing measure should be avoided where possible because tracheotomies can extend the stenosis and compromise the length of normal trachea available for surgical anastomosis. If a presurgical tracheotomy is indeed needed, it should be placed in the area of stenosis or at the site of any previous tracheotomy. Then both the stenosis and the new stoma can be resected together during subsequent tracheal surgery. Any temporizing tracheostomy tube must pass the entire length of the stricture to provide a secure airway.
Patients who are not in overt respiratory distress are managed by providing supplemental oxygen with humidification, intensive monitoring, gentle chest physiotherapy, and oropharyngeal suctioning. Nebulized saline, N-acetyl cysteine, and other agents to improve mucociliary clearance have been used, but they carry the risk of provoking bronchospasm (Fig. 26.2). Often, patients settle sufficiently with the gentle use of anxiolytics to enable a semi-elective bronchoscopic evaluation before definitive treatment can be instituted.
Evaluation
Clinical Evaluation
The clinical presentation is dependent on the severity of airway obstruction and on cardiopulmonary reserve. Typically, patients remain asymptomatic until stenosis exceeds 50%. Effort tolerance is first affected because the pressure drop across any obstruction increases with increasing airflow velocity. Symptoms at rest often only present when tracheal lumen narrowing is ≤5 mm. As a result of being relatively asymptomatic despite the presence of severe airway obstruction, these patients may only be first identified when a precipitant such as infection, edema, or mucous impaction triggers a life-threatening airway occlusion.
A high index of suspicion is needed if the patient presents with a history of prior endotracheal intubation or tracheotomy, tuberculous endobronchitis, gastroesophageal reflux disease, or systemic illnesses such as Wegener’s granulomatosis or relapsing polychondritis. Wheezing refractory to bronchodilator therapy and recurrent pneumonia are classical presentations, and stridor or unilateral wheezing on physical examination is a clear indication for airway evaluation. Hypoxia or hypercapnia may not be present even in the presence of severe dyspnea.
Investigations
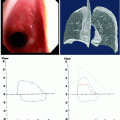
Computed tomography (CT) can provide valuable anatomical information on the location and extent of the lesion. However, standard axial imaging provides limited information on the length of obstruction, the relationship to adjacent mediastinal structures, and the presence of malacia. Therefore, multi-planar imaging with three-dimensional reconstruction and dynamic expiratory CT is preferred (Fig. 26.3).
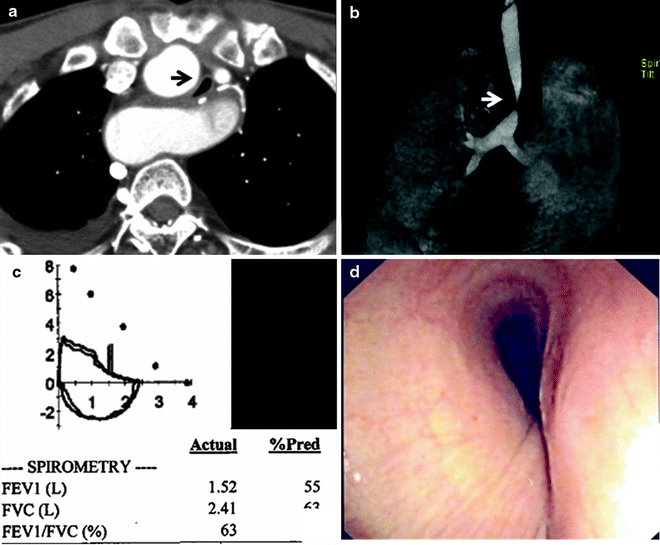

Fig. 26.3
Evaluation of airway stenosis with (a) computed tomography, (b) three-dimensional reconstruction imaging, (c) spirometry, and (d) flexible bronchoscopy showing a mid-tracheal extrinsic compression due to a vascular sling
Spirometry may yield classical flow-volume loop patterns of upper airway obstruction (Fig. 26.3), but sensitivity is low, and the typical flow loop cutoffs are not seen until the tracheal lumen is narrowed to <10 mm. Hence, normal spirometry does not rule out the diagnosis of airway obstruction in any patient in whom it is suspected and bronchoscopic evaluation is still warranted. Although there may be some role for spirometry in objectively documenting physiological severity of lesions, this evaluation can be confounded by obstructions at multiple sites and by comorbid airway disease such as chronic obstructive pulmonary disease (COPD). Furthermore, spirometry is a demanding investigation on patients who are in respiratory distress, and they are often unable to produce either satisfactory or reproducible measurements.
Bronchoscopic Evaluation
Bronchoscopy is the diagnostic modality of choice in all airway obstruction. However, the timing is debatable with some endoscopists recommending initial, routine flexible bronchoscopic evaluation of all referred cases as opposed to deferring airway inspection to the time of treatment with rigid endoscopy. Flexible bronchoscopy may be dangerous in patients with severe airway obstruction because the obstruction can be worsened by the bronchoscope physically occluding the limited airway. However, this problem can be overcome in some cases by using ultrathin or pediatric scopes. Respiratory failure may also be exacerbated by moderate sedation. Therefore, any attempt at bronchoscopy must be undertaken in a setting with a skilled endoscopist, appropriate personnel, and facilities for advanced airway management including emergency tracheotomy. Any doubt about the ability to secure the airway should prompt evaluation with a rigid ventilating bronchoscope that has the advantage of enabling the endoscopist to concurrently control ventilation and examine the airways. Larger instrumentation is also possible with more options for therapeutic intervention, and flexible bronchoscopy can still be performed via the rigid barrel to examine the lobar and segmental airways.
Bronchoscopy enables detailed evaluation of lesions, patency assessment of airways distal to the obstruction, and examination of mucosa for evidence of active inflammation. Biopsies can be taken if the diagnosis is uncertain. However, bleeding from endobronchial biopsy, which is usually self-limiting, can in some instances exacerbate airway occlusion. Rigid bronchoscopy provides a safer option for the biopsy of central vascular lesions by securing the airway, offering superior suction capability, and enabling direct tamponade of any bleeding sites.
A standard classification system for airway obstruction has yet to be established, and this can prove a major hindrance to communication in a multidisciplinary team. One recently proposed system by Freitag et al. improves precision and interobserver agreement by classifying stenosis as either structural or dynamic and then further subdivides lesions by location, degree of stenosis, and transition zone. Although other staging systems of laryngotracheal stenosis have been previously devised and correspond with surgical outcomes, these systems are heavily weighted to the location of the lesion and are limited in application to only the upper trachea.
In the classification system, structural stenosis is classified into four groups: intraluminal occlusion, extrinsic compression, distortion, or scar (Fig. 26.4). Dynamic stenosis is defined by >50% airflow obstruction in expiration and is categorized as either cartilage malacia or a floppy posterior tracheal membrane (Fig. 26.5). Cartilage damage results in a saber-sheath trachea if the lateral walls are weak, a crescent-shaped trachea if the anterior walls are weak, and a circumferentially occluded trachea if both anterior and lateral walls are involved. In contrast, excessive dynamic airway collapse involves only the bulging of the floppy posterior wall. It typically involves the lower trachea and main stem bronchi. Endoscopic quantification of the degree of any dynamic stenosis needs to account for confounding variables such as patient position, depth of sedation, and the use of respiratory maneuvers in functional bronchoscopy.
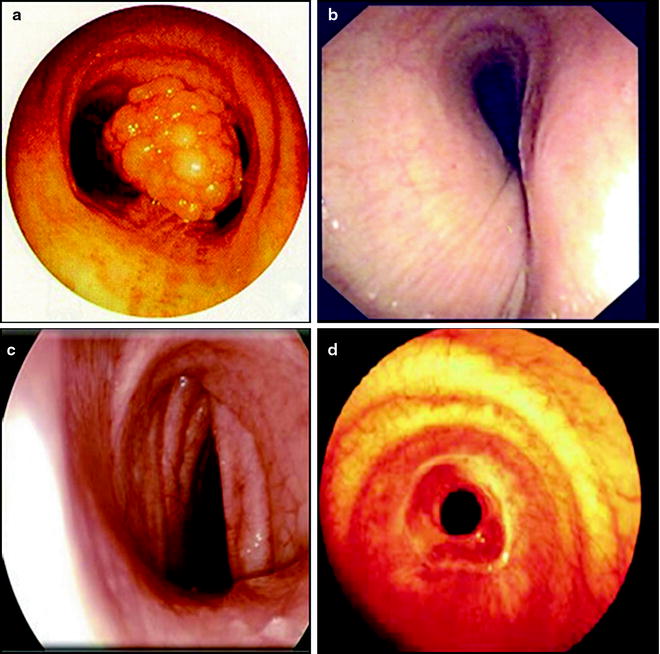
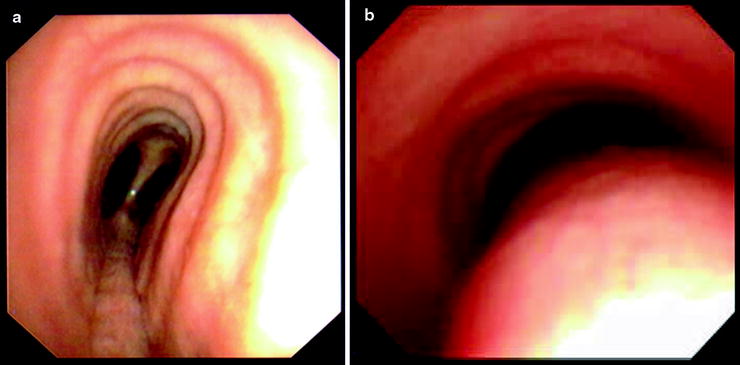

Fig. 26.4
Classification of structural airway stenosis: (a) intraluminal occlusion from papillomatosis, (b) extrinsic compression from a vascular sling, (c) distortion in an A-shaped post-tracheotomy stricture, and (d) scar from idiopathic tracheal stenosis

Fig. 26.5
Dynamic airway obstruction showing (a) saber-sheath trachea with lateral wall damage and (b) excessive dynamic airway collapse with a floppy posterior membrane
The location of the pathology is described to be in the upper, middle, or lower third of the trachea or in either main stem bronchi (Fig. 26.6). The surgical implication is that up to half the upper and middle trachea can be resected, but the length of resection is limited to 40 mm at the level of the carina. Measurements in relation to normal airway landmarks such as the vocal cords, cricoid cartilage, carina, and major bronchial bifurcations provide greater accuracy in defining location. These measurements should be made from the top, as well as from the bottom of the lesion. A lesion is considered a simple stenosis if confined to a single cartilage ring without any associated malacia or chondritis. Complex stenoses are more extensive in length (Fig. 26.7) and are complicated by cartilage damage. They often have poorer results with endoscopic intervention.
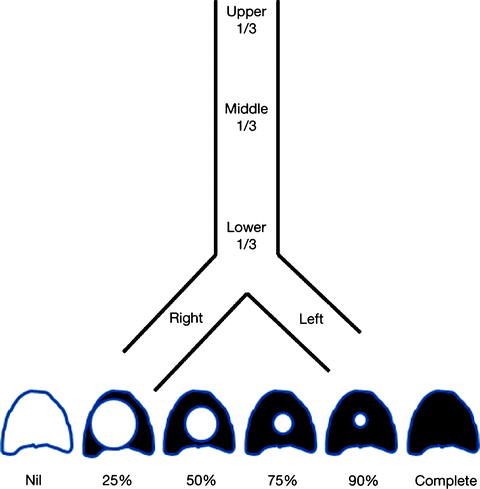
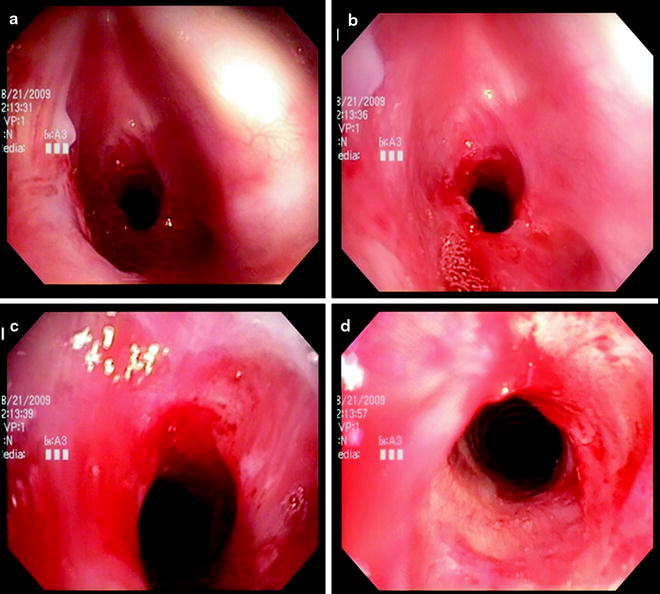

Fig. 26.6
Worksheet representation of location and degree of stenosis

Fig. 26.7
Evaluating a complex subglottic stenosis moving from the proximal portion (a) of the lesion to the most distal portion (d)
The degree of endoluminal occlusion is categorized as <25%, 26–50%, 51–75%, 76–90%, and 90% to complete obstruction (Fig. 26.8). The severity is measured at its worst point in the respiratory cycle, and this usually occurs at the end of expiration. Transition zone refers to the abruptness of change of the airway obstruction which can be sudden as in web stenosis or more gradual and shaped like a bottleneck in cicatricial lesions (Fig. 26.9).
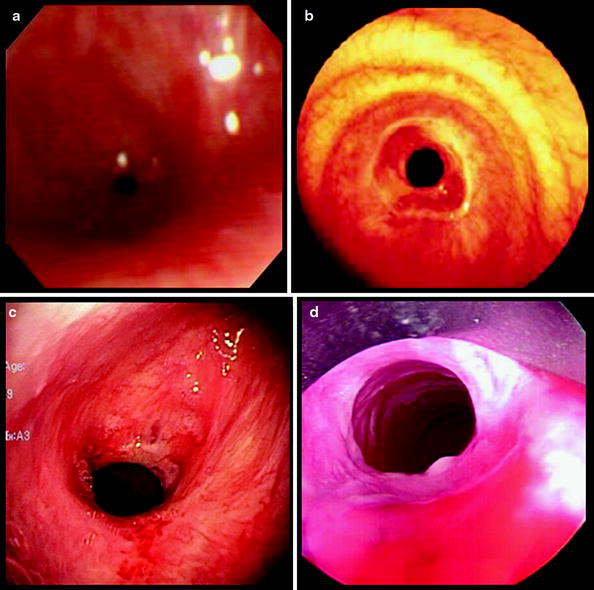
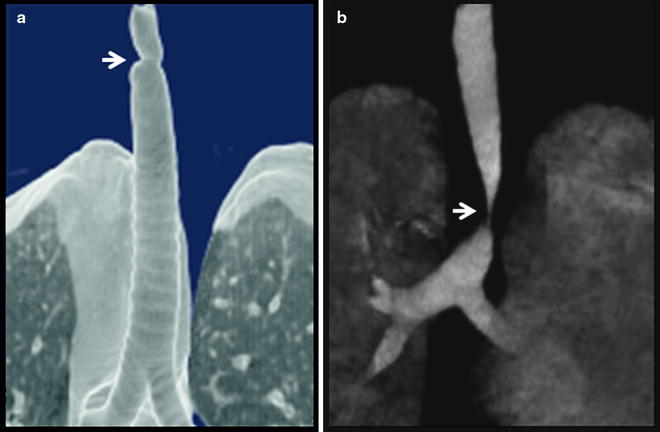

Fig. 26.8
The degree of endoluminal occlusion as visualized on flexible bronchoscopy as (a) 90%, (b) 75%, (c) 50%, and (d) 25%

Fig. 26.9
Three-dimensional reconstruction computed tomography imaging showing (a) abrupt and (b) tapered transition zone
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree