Thymic Hyperplasia
Also known as thymic rebound, thymic hyperplasia represents enlargement of the thymus after a period of atrophy induced by medications or severe illness. In the setting of chemotherapy, the thymus atrophies in 90% of patients.
2 After the illness has subsided or the medication administration has ceased, the thymus slowly recovers in volume with the total size possibly exceeding its baseline value. Imaging clearly demonstrates this pattern of involution and subsequent regrowth.
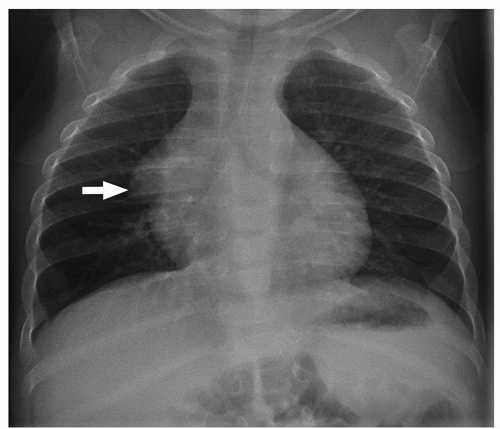
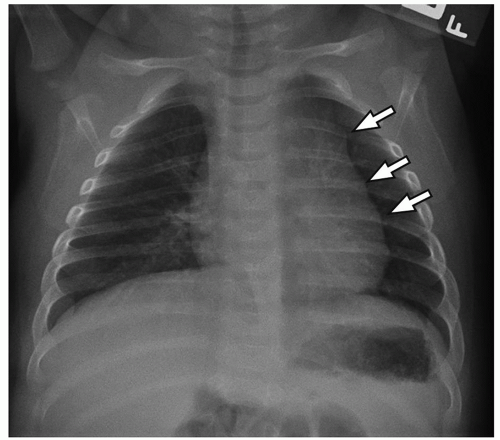
On chest radiographs, the thymic shadow significantly diminishes in size or completely disappears during the associated stressor. Once the illness or medication stress has subsided, the thymic shadow rebounds and may demonstrate lobulated and convex contours, which can be disconcerting for the imager. Similarly, cross-section imaging demonstrates thymic hyperplasia to best advantage (
Fig. 11.6). The hyperplastic thymus typically demonstrates homogeneous soft tissue attenuation and signal on CT and MRI, respectively, helping to differentiate it from more ominous processes. On PET imaging, thymic rebound is quite frequently encountered and should be distinguishable from persistent or recurrent neoplasm. In addition to uniform soft tissue attenuation on co-registered CT, homogeneous rather than masslike focal uptake is a supporting finding for thymic rebound on PET. Additionally, the rebounding thymus typically should not have a standardized uptake value (SUV) of more than 4.
2No specific treatment and follow-up is needed for thymic hyperplasia other than distinguishing it from more ominous diseases.
Lymphoma (Hodgkin and Non-Hodgkin Lymphoma)
Lymphoma represents the most common anterior mediastinal mass in childhood, arising from abnormal proliferation of lymphocytes.
1,
2 Lymphoma is the third most common malignancy in children with only leukemia and central nervous system tumors being more common. Traditionally, lymphoma is subdivided into Hodgkin, histologically characterized by Reed-Sternberg cells and variants (
Fig. 11.10), and non-Hodgkin lymphoma (NHL), resulting from clonal proliferation of lymphocytes (
Table 11.2).
Hodgkin lymphoma in children typically occurs after the age of 10 years. It more often affects boys than girls by a ratio of 2:1.
31 It is divided into classical Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma. In classical Hodgkin lymphoma, Reed-Sternberg cells and variants are the characteristic malignant cell (
Fig. 11.10); they are present in a background rich in benign lymphocytes and other inflammatory cells with variable amounts of fibrosis. Nodular sclerosis subtype, most often showing prominent interspersed fibrous bands, is the most common subtype in the pediatric mediastinum. Nodular lymphocyte-predominant Hodgkin lymphoma is rare in children.
32 Generally, Hodgkin lymphoma carries a good prognosis with a cure rate of ˜90%.
2,
31 The majority of affected pediatric patients present with chest pain and discomfort related to enlarged mediastinal lymph nodes with associated compressive effects on the adjacent airway and vascular structures. However, affected children occasionally present with asymptomatic cervical or axillary lymphadenopathy. Additional signs and symptoms include fever, night sweat, and unexplained weight loss. These constitutional symptoms are known as B symptoms, and they affect the disease staging. Based on the Ann Arbor staging classification, both Hodgkin lymphoma and NHL (discussed below) are categorized into four stages (
Table 11.3).
33NHL, which is more common than Hodgkin lymphoma, typically presents in the first and second decades of life but generally occurs in the pediatric patients younger than 5 years of age.
1,
6,
31 Similar to Hodgkin lymphoma, boys are affected more commonly, with a male-to-female ratio of 3:1.
31 Four subtypes of NHL are particularly prevalent in children: (1)
lymphoblastic lymphoma (most commonly with a T-cell phenotype) (
Fig. 11.11), (2) Burkitt lymphoma, (3) diffuse large B-cell lymphoma (
Fig. 11.12), and (4) anaplastic large cell lymphoma. With T-cell lymphoblastic lymphoma, affected pediatric patients commonly present with a mediastinal mass. In contrast, Burkitt lymphoma commonly presents in the abdomen, most frequently around the terminal ileum. Diffuse large B-cell lymphoma presents variably and may involve the mediastinum, often with fibrosis that imparts nodularity.
31,
34 Symptoms of mediastinal NHL are often related to compression or obstruction of adjacent airway and vascular structures.
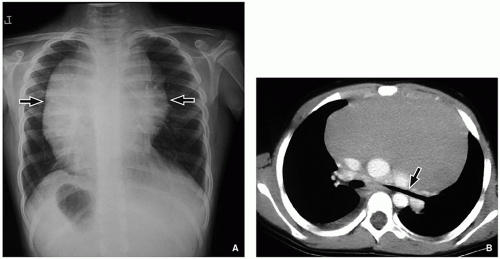
Imaging features of pediatric lymphoma rely heavily on the underlying histology. For Hodgkin lymphoma, chest radiographic findings are variable, ranging from completely normal with minimal mediastinal lymph node enlargement to a very large anterior mediastinal mass (
Fig. 11.13A). Following chest radiography, cross-sectional imaging is typically
performed, usually contrast-enhanced chest CT (
Fig. 11.13B). Here, the CT serves the purpose of confirming the presence of an anterior mediastinal mass while allowing for the assessment of adjacent nodal involvement, which helps in disease staging (
Table 11.3). Imaging appearance of affected nodes ranges from small individually discernible lymph nodes most commonly in the prevascular and pretracheal region to large or conglomerated, lobulated masses that displace adjacent mediastinal structures.
2,
31 Hodgkin lymphoma demonstrates variable signal characteristics on MRI, although involved lymph nodes usually have hyperintense signal on T2-weighted and intermediate signal on T1-weighted MR images.
Over the past several years, PET and PET/CT are transitioning into the forefront for lymphoma imaging as they provide useful physiologic data in addition to traditional anatomic evaluation. Active lymphoma demonstrates marked increased FDG activity (
Fig. 11.14) relative to the mediastinal blood pool, whereas fibrosis and necrotic tissue in the region of prior disease demonstrates no appreciable FDG activity.
Unlike HL, the site of involvement of NHL is more variable than that of HL; common sites include the abdomen, thorax, and head and neck. Thoracic involvement occurs in ˜50% of cases.
2,
31 On chest radiograph, thoracic NHL presents as a large anterior mediastinal mass with convex outer margins and often displacement of the mediastinal structures such as mediastinal vessels and trachea (
Fig. 11.15A). Discrete or large conglomerated mediastinal lymphadenopathy may be seen on CT and MRI. With contrast administration, NHL may demonstrate heterogeneity (
Fig. 11.15B) or central irregular nonenhancing regions representing focal areas of necrosis. Adjacent hilar and subcarinal lymph node chains are frequently involved. Also, pleural-based masses or effusions may be seen, a feature not commonly seen with Hodgkin lymphoma. Like Hodgkin lymphoma, PET is particularly helpful in the assessment of staging, disease activity, and treatment response. Particularly instructive is PET’s ability to discriminate viable tumor from scar and its ability to detect disease within otherwise normalappearing lymph nodes.
Treatment for both Hodgkin lymphoma and NHL relies primarily on chemotherapy, sometimes with concurrent radiation therapy. After treatment, coarse calcification at the site of prior disease may develop (
Fig. 11.16). Comparatively, the cure rate is higher for Hodgkin lymphoma at 90% with a cure rate among pediatric patients with NHL being 80%.
31,
35 For NHL, bone marrow transplantation may also be utilized as a treatment option.