Mediastinum: The Mediastinum
The mediastinum is the central space of the thorax located between the two pleuropulmonary cavities to the right and left, the cervicothoracic inlet above, and the interdiaphragmatic thoracoabdominal outlet inferiorly. It contains the heart and great vessels, the thymus, the esophagus, the trachea and main bronchi, lymph nodes, and mediastinal pleural reflections, as well as the vagus and phrenic nerves.
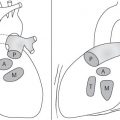
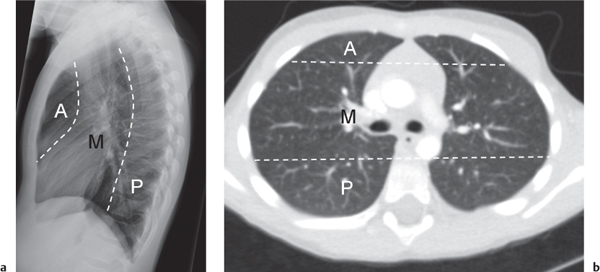
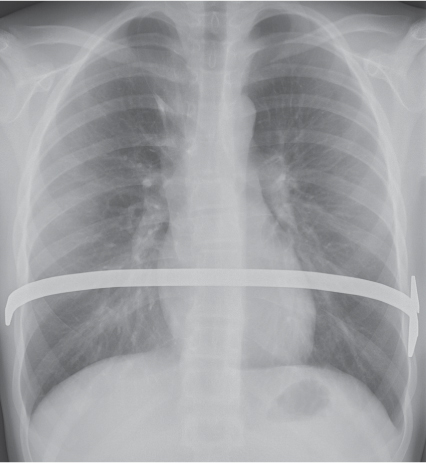
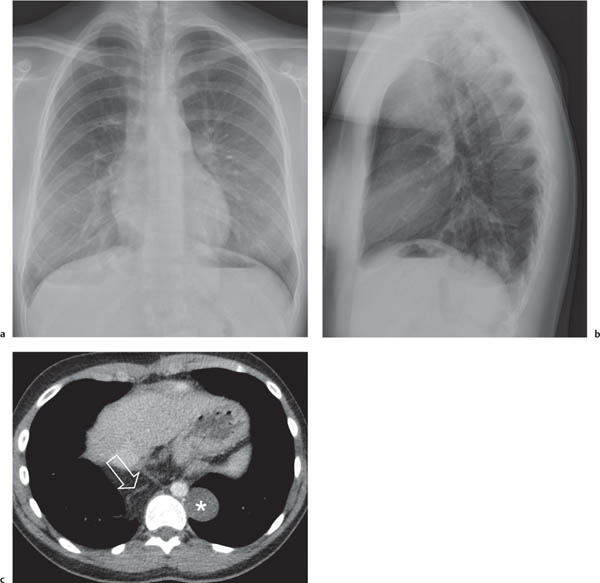
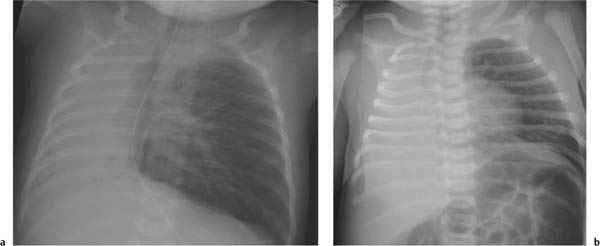
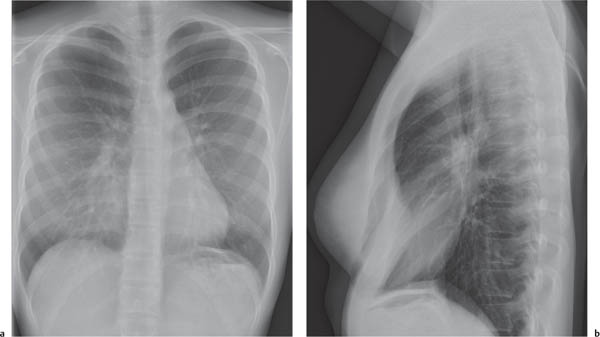
On the lateral thoracic radiographs, the mediastinum is divided into the anterior, middle, and posterior compartments ( Fig. 1.161a ). The anterior mediastinum is located between the sternum and the pulmonary root, and the posterior mediastinal space extends posteriorly from a line connecting the anterior surfaces of the thoracic vertebral bodies. The middle mediastinum is the remaining portion.
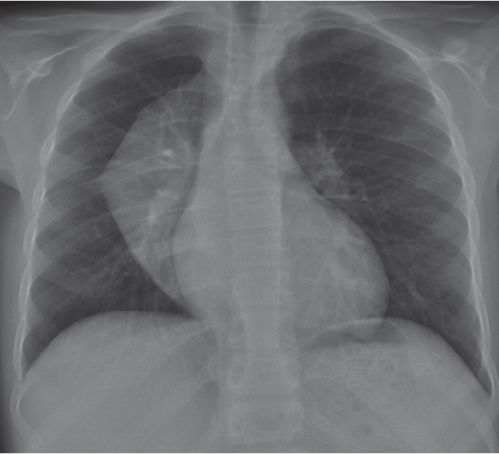
On the frontal thoracic radiograph, the mediastinum is divided in the superior, middle, and inferior mediastinum ( Fig. 1.161b ). The superior mediastinum extends from the thoracic inlet to the superior aspect of the hila. The middle mediastinum comprises the hilar region. The inferior mediastinum is bordered by the lower aspect of the hila and the diaphragm.
This division of the mediastinal space is used for putting together a differential diagnosis. Keep in mind, however, that lesions arising in one mediastinal compartment often involve an adjacent one, which makes it sometimes difficult to define the originating site.
Neonates and Infants
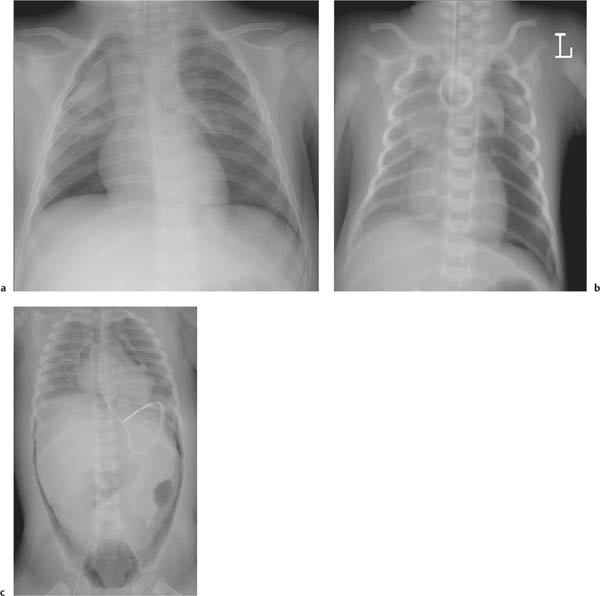
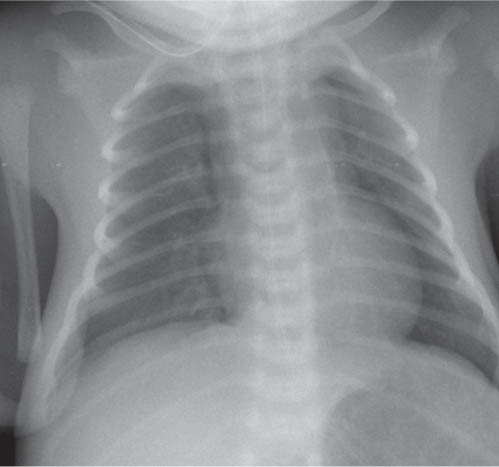
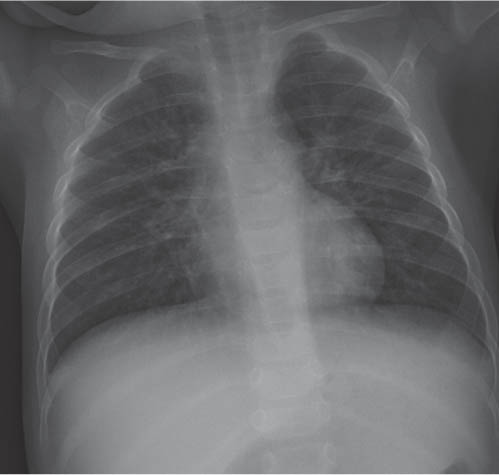
The superior and often the middle mediastinum are physiologically widened because of the presence of the thymus. When the thymus shrinks or disappears, as a consequence of stress, the mediastinum will appear narrow. The thymus rebounds after relief of the stress. The shape and size of both the normal as well as the rebounding thymus is highly variable from patient to patient.
Older Children
The thymus gradually involutes. By the age of 3 years, the thymus is usually not border-forming on the frontal chest radiograph.
School-aged Children
The mediastinum assumes the size and contours as in adults.
Imaging Techniques
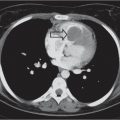
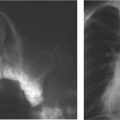
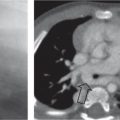
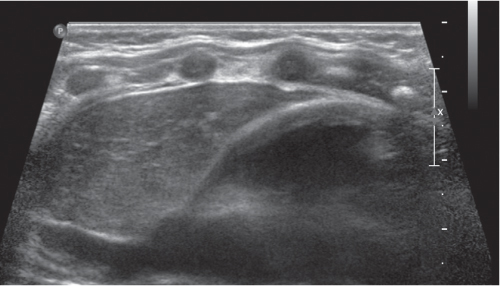
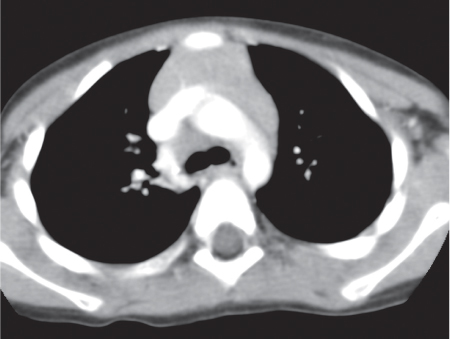
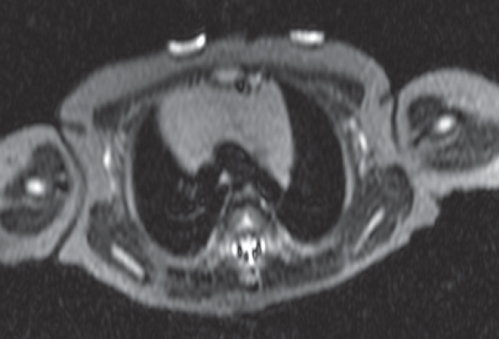
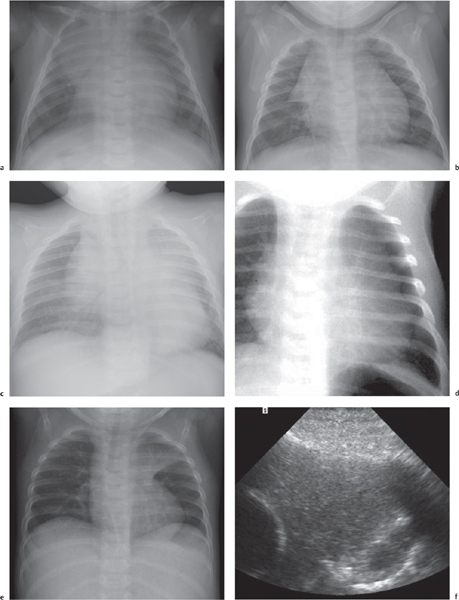
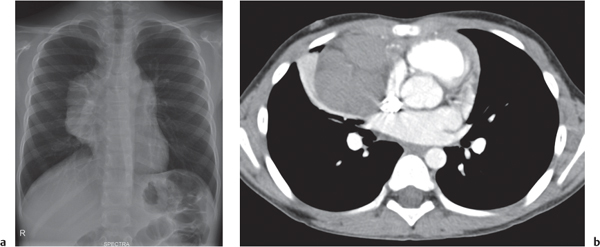
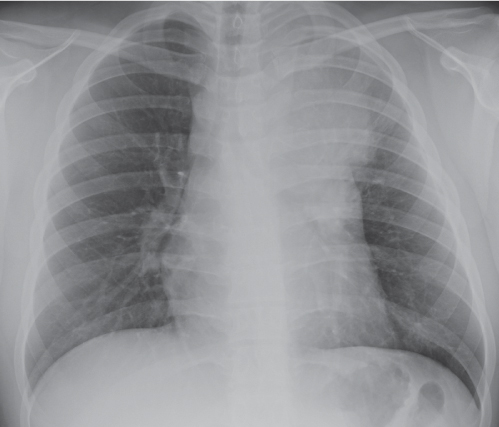
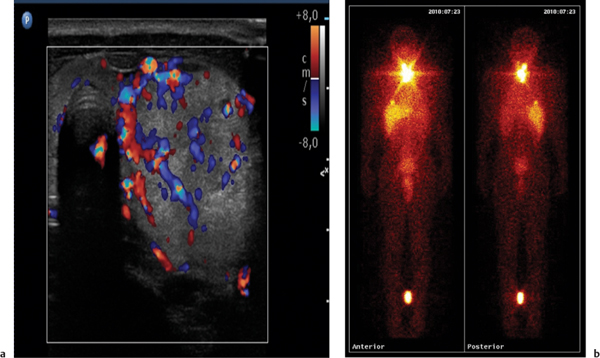
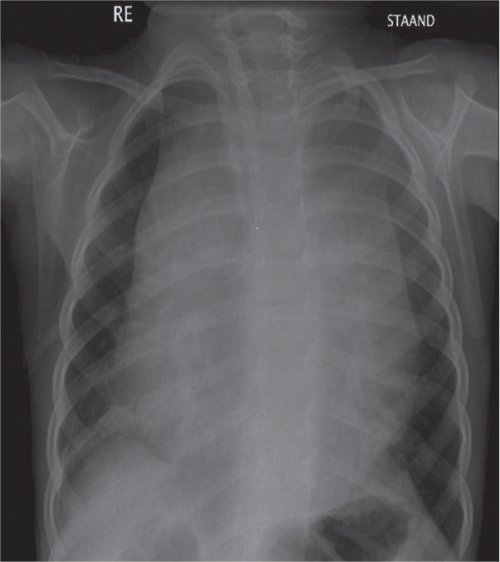
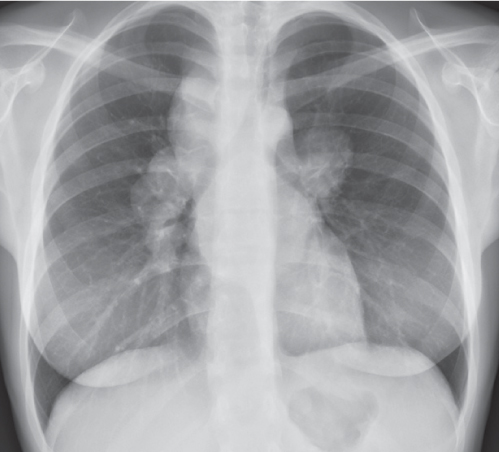
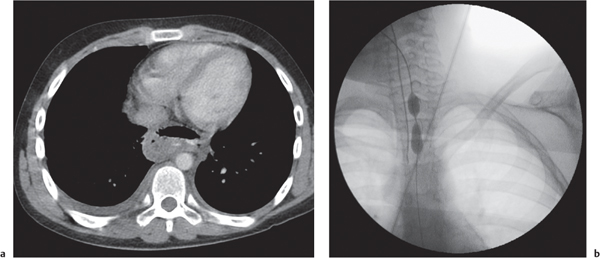
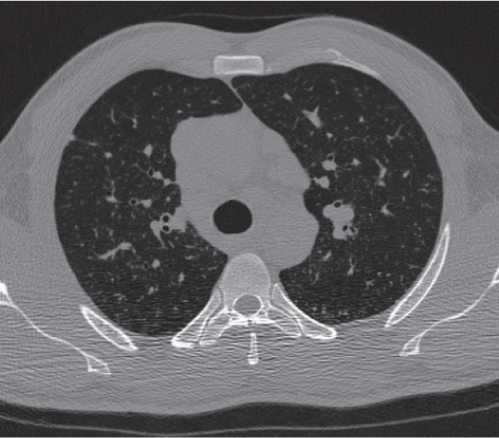
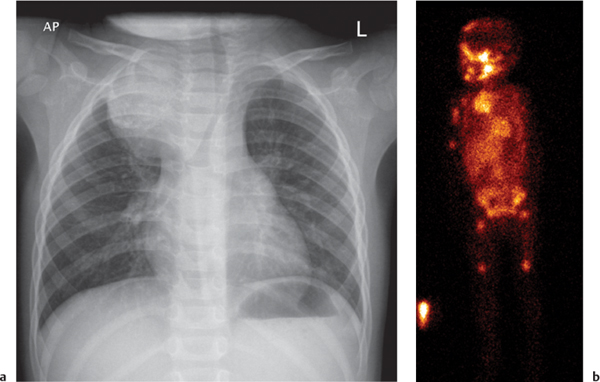
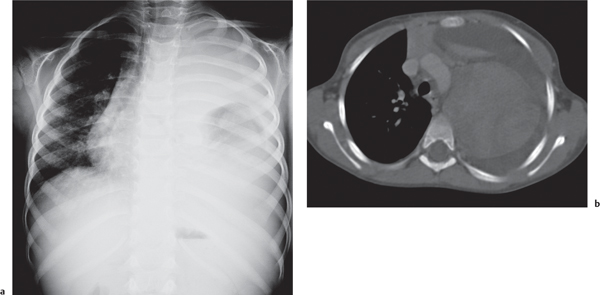
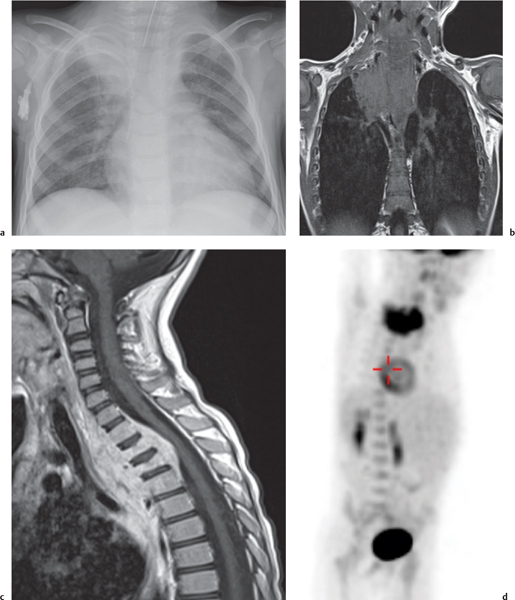
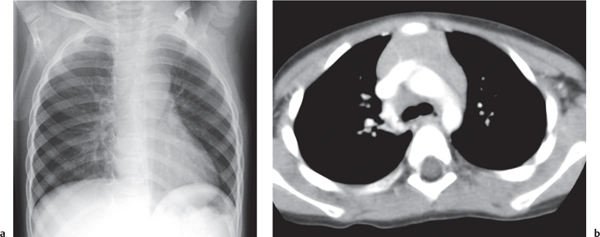
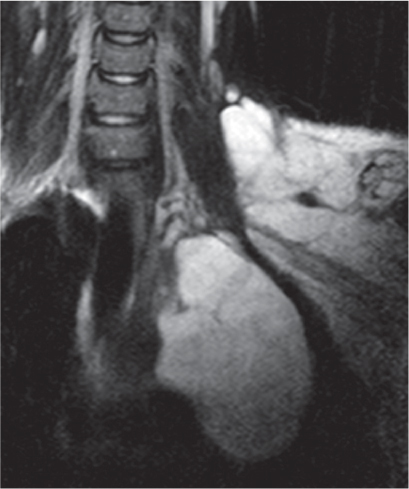
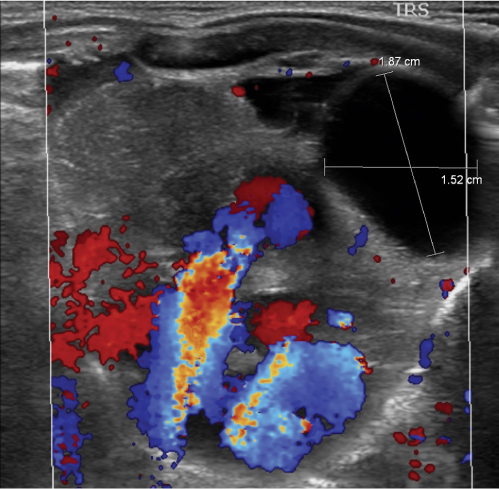
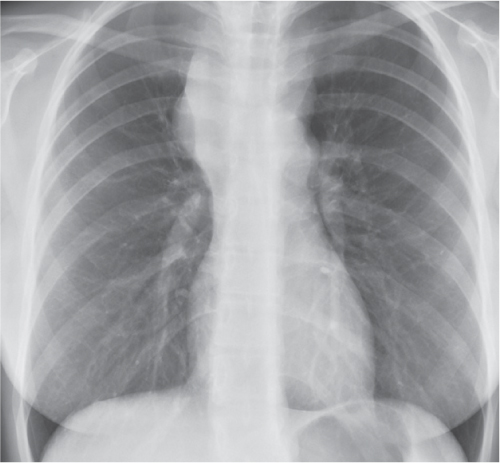
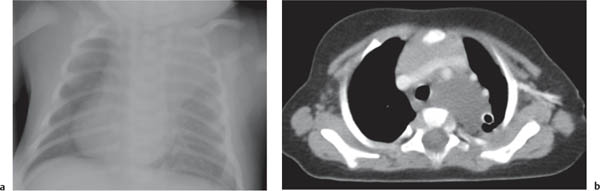
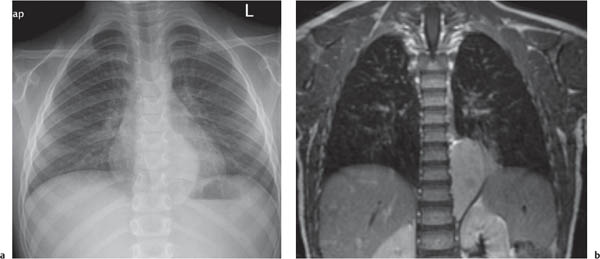
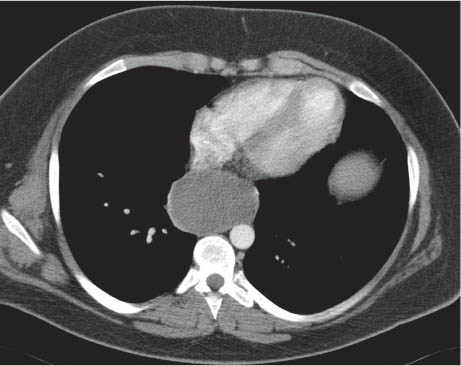
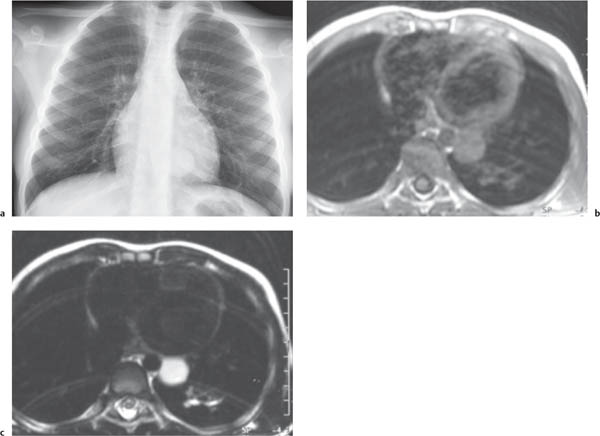
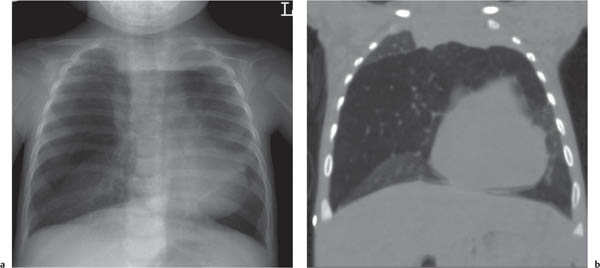
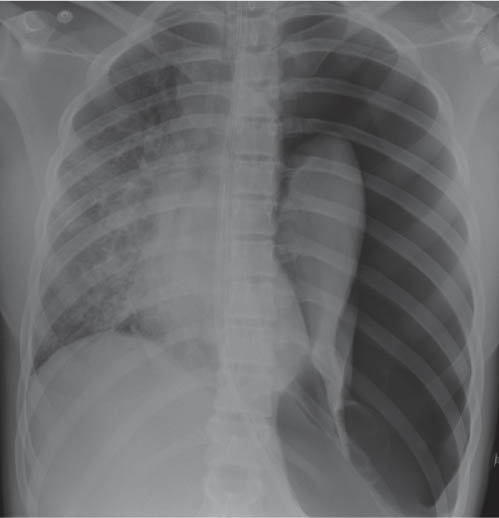
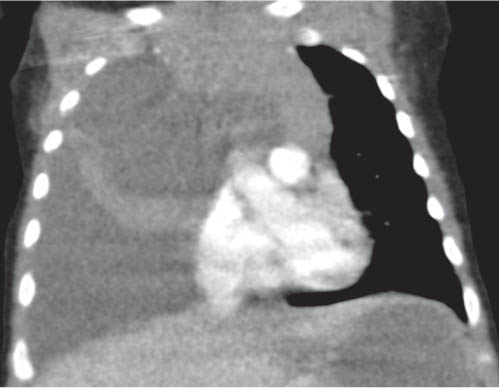
This space can be examined using anteroposterior and lateral thoracic radiographs ( Fig. 1.162 ), US ( Fig. 1.163 ), CT ( Fig. 1.164 ), and MRI ( Fig. 1.165 ).
In early childhood, the mediastinum does lend itself to US examination. There are three main approaches to the mediastinum: (1) suprasternal approach allows examination of the superior mediastinum; (2) right and left parasternal approach, with the patient in lateral decubitus, explores the anterior mediastinal compartment and the heart; (3) subcostal abdominal approach allows transdiaphragmatic investigation, which is useful for masses of the cardiophrenic angles.
MRI is superior to CT for evaluating extension of lesions, in particular of tumors invading the spinal canal and those in contact with the heart or the cervicothoracic or thoracoabdominal junction.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree