, Valery Kornienko2 and Igor Pronin2
(1)
N.N. Blockhin Russian Cancer Research Center, Moscow, Russia
(2)
N.N. Burdenko National Scientific and Practical Center for Neurosurgery, Moscow, Russia
Melanoma is the most malignant tumor that develops as a result of malignant transformation of pigment cells—melanocytes located in various body tissues. The first mention of a “fatal black tumor with metastases and a black fluid in the body” appeared in European literature between 1651 and 1760. The term “melanoma ” was first proposed by Carswell in 1838.
The incidence of melanoma in the world is more than 230,000 people annually. In the USA, more than 32,000 patients are diagnosed with melanoma each year, of whom 7200 annually die (Balch and Kirkwood 1997); in Russia this figure approaches 9000. In recent decades, a steady increase in the incidence of melanoma in all regions of the world is observed, with the tumor being more common in young people.
Melanoma is the third by frequency of metastases to the brain, although many authors put it on the second place, between lung cancer and breast cancer (Stehlin et al. 1965; Romodanov 1973; Hauward and Hayward 1976; Bullard and Cox 1981; Johnson and Smith 1995). According to Greenlee et al. (2001), melanoma metastases constitute 5–21% of the total cases of secondary brain malignancies, in spite of the fact that melanoma proportion in the structure of malignant tumors is only 4%. In our clinical material, melanoma metastases in the brain occurred three times less often (about 18.2%) than lung cancer metastases.
The mean interval between the diagnosis of melanoma and the development of MTSs in the brain is 1.2–3.5 years (Chason et al. 1963; Markesbery et al. 1978; Zimm et al. 1981; Damek 2003). When the size of brain tumor is <5 mm, survival of melanoma patients after surgery is 89–100%. When melanoma metastasizes in the internal organs, the 5-year survival rate is less than 10% (Rigel et al. 2000). The prognosis for patients with metastatic melanoma in the brain is the most unfavorable. The tumor volume, depth of invasion, ulceration, satellites, site, and radical treatment have a key prognostic value.
According to Rhodes et al. (1987), there is a definite relationship between the intensity of metastasizing and the age of melanoma patients. In children, melanoma metastases are rare. The authors noted the maximum intensity of metastasizing by 30–40 years of age; patients had metastases at various sites as early as after 3–4 months. A decrease in the tendency to metastasize was observed in elderly patients (Chao et al. 2004).
According to Akimov and Gershanowitch (2001), melanomas metastasize within the first months after the onset of the disease, while uncontrolled dissemination of the tumor was determined in many patients already at the time of diagnosis. According to McWilliams et al. (2008), metastases were detected at necropsy in 50–70% of patients diagnosed with melanoma, and only 60% of these cases of metastases in the brain were detected during their lifetime.
There are primary and metastatic melanomas in the brain. Primary melanoma in the central nervous system is very rare—1% of cases (Farrokh et al. 2001)—and is a surgical or necropsy finding. Primary melanoma usually develops in the choroidal plexus or the pial membrane of the fourth ventricle, or around the brainstem (especially in the ventral regions), as well as in the upper sections of the spinal cord, since these areas contain the greatest concentration of melanocytes (Gebarski and Blaivas 1996; Arbelaez et al. 1999). Primary melanoma usually metastasizes to the meninges, rarely affecting the parenchyma (Rubino et al. 1993; Arbelaez et al. 1999). Often primary melanoma spreads along the meninges after surgery. van der Ree et al. (1999) noted that, with the tumor located in the posterior fossa, 33% of patients experienced involvement of not only intracranial meninges but also of the spinal cord 2–13 months after the surgery.
In secondary metastatic brain lesions, primary melanoma is usually in the skin (97%), while in 2–3%, melanoma is detected in the vagina, anorectal area, choroid and iris, penis, and lungs. Melanoma metastases can also be found in the lymph nodes of the abdominal and thoracic cavities, lungs, gastrointestinal tract, adrenals, and heart. According Mogila (1979), melanoma metastases were found in the heart muscle in 9.4% of cases and in the adrenal tissue in 10.4%. The tumor lesions in the adrenal glands result in a decrease in the corticosteroid function up to the complete failure of this organ, which is often the cause of rapid deaths. According to the same author, the brain was the only place of melanoma metastases in 51.9% of patients.
Melanomas metastasize to the brain via both the hematogenous and lymphogenous routes Babchin et al. 1974a, b; Iconography et al. 1977). According to Martynov et al. (2002), melanoma cells enter the brain more often via the hematogenous route, through the arterial circle. Lymphogenous spread of melanoma metastases to the brain is possible, when the primary pigmented tumor is located in the skin of the head and neck. Zhuravlev et al. (1994) noted that metastases usually first appear in the regional lymph nodes, i.e., local lymphogenous spread of tumor cells occurs earlier than hematogenous one. However, other researchers believe, referring to the appearance of tumor cells in the regional lymph nodes and distant organs, that metastasizing occurs via a mixed route, i.e., both lymphogenically and hematogenically.
By their morphological structure, melanoma metastases in the brain, as compared with other metastases, most resemble the primary pigmented tumor. Just as in primary melanoma, metastases contain different histological variants of epithelium-like, spindle cell, and mixed structures. Metastases may differ from primary melanomas by the amount of melanin in the direction of both its increase and decrease (Ganina et al. 1978). In separate cases, amelanotic melanoma metastasized and became pigmented and vice versa.
Amelanotic (nonpigmented) forms of melanoma may still show some amount of melanin. However, according to Golbert (1975) and Tiraspolskaya (1975), a complete lack of melanin allows to suspect melanoma based on polymorphism of the tumor tissue, typical of this tumor, in combination with sarcomatoid or epithelioid cell structure. Both metastatic and primary CNS melanomas can be amelanotic. According to Ganina and Naleskina (1991), nonpigmented or weakly pigmented forms of melanomas have a pronounced tendency to metastasize and a poor prognosis. If primary melanomas remain undetected, according to Ikonopisov et al. (I977), the main reason for that is not only a microscopic tumor size but also its amelanotic form.
Metastases of melanoma in the brain are characterized by frequent hemorrhages(Hauward and Hayward 1976; Mogila 1979; Konovalov et al. 1997a, b, c; Karp et al. 2002). The main causes of hemorrhages in the tumor tissue are the degree of the tumor anaplasia and specifics of tumor vascularization. The clinical course of the disease in such cases drastically worsens.
On direct angiograms, manifestations of melanoma metastases in the brain are characterized by the “porous structure” with sharp edges and an abundant vascular pattern, and at the same time, an expressed dislocation of brain vessels is observed. It should be noted that, in some rare cases, pigmented tumors by their structure may resemble glioblastoma with formation of arteriovenous shunts on angiograms.
In contrast to metastases of other primary tumors to the brain, with iso- and/or hypodense characteristics, cerebral metastases of melanoma often have a heterogeneous density on CT due to frequent hemorrhages. In our experience, in 76% of cases of cerebral melanoma MTSs, the solid part of the tumor tissue was identified as a rounded site with moderately high density, and CA administration only enhanced its density characteristics. Our data correlate with data of other authors (Weisberg 1985; Patten et al. 1990; Ho Shon et al. 2008; Goulart et al. 2011). Thus, Weisberg (1985) noted using native CT in patients with metastatic melanoma that melanoma lesions were characterized by a hyperdense signal in 75%, while a hypo- and isodense signal was determined in 23% and 3%, respectively. It can be argued that CT manifestations of melanoma metastases in the brain are associated not only with the specifics of the tumor histogenesis but also with hemorrhages to its tissue, due to which the tumor density increases.
In case of detection in the brain of multiple tumor lesions with an increased density during a CT study, melanoma metastases should be suspected even if the primary tumor has not yet been detected. In terms of differential diagnosis in cases where there are hyperdense characteristics of the lesions on native CT, non-tumor hemorrhages should be first ruled out.
Even small metastatic melanomas are characterized by a pronounced perifocal edema.
As a result of CT perfusion studies, we noted that the tissue of melanoma metastases has one of the highest blood volume values—CBV = 15.03 ± 9.7 ml/100 g—only slightly inferior to that of kidney cancer metastases. Of note is a large dispersion of CBV values in melanoma, which is probably related to different degrees of tumor capillary network depending on its size and “age.” These findings are supported by the fact that melanoma metastases are characterized by the highest values of the capillary blood flow in the tumor depth: CBF = 113.99 ± 24.25 ml/100 g/min. Melanoma metastases passed the blood through their parenchyma the fastest: they were characterized by the lowest MTT values (7.22 ± 2.1 s) in our group.
Our experience with CT perfusion techniques in the diagnosis of metastatic brain lesions allows to establish its high efficiency in the determination of perfusion characteristics of metastatic tumors, depending on the primary source and reliably differentiate melanoma metastases from metastatic lesions of other origin with similar CT manifestations during a native CT study (p < 0.03).
As noted earlier, a CT perfusion method was used not only in primary melanoma diagnosis but also in the monitoring of treatment effectiveness in radiation therapy, often revealing complications during the treatment. CT perfusion in some cases allowed us to unambiguously identify a residual tumor on the background of a hemorrhage, having similar manifestations on routine CT and MRI.
Similarly to other metastatic tumors, melanoma metastases tend to grow as nodular lesions without any signs of invasion of the surrounding brain substance, unlike primary malignant brain tumors that diffusely infiltrate adjacent tissues.
Melanoma metastasizes in any portion and structure of the brain and skull. The most common site is the brain. However, metastatic lesions may occur in the dura mater, choroid plexus, pituitary gland, and pineal gland. In our material, melanoma metastases were detected in the orbit.
Metastatic melanoma has a number of characteristic pathognomonic MRI manifestations that allow to assume the nature of the primary tumor with a greater probability, even based on routine MRI studies. It is known that melanin is characterized by the presence in its structure of unpaired electrons and has paramagnetic properties. This gives melanoma its characteristic signs in MR imaging, manifested as an enhanced MRI signal in T1-weighted MRI, even before the CA administration(Atlas et al. 1987a, b; Isiklaret al. 1995; Escott 2001; Breckwoldt et al. 2015). In our material, homogeneous metastatic melanomas (without signs of central necrosis) in T2-weighted images in 60% of cases had moderately hypointense or hypointense signals and in 31% of cases had iso-hypointense signals. In case of a tumor hemorrhage, the character of MR signal on T1-weighted MRI changed from hyperintense to hypointense depending on the “age” and the number of hemorrhages. Hemorrhages to the tumor tissue were detected in 60% of cases and were often identified both with single and multiple metastases of melanoma.
A decrease in the signal on T2-weighted MRI is typical not only for melanoma metastases (91%): colon cancer metastases were hypointense in 83% of our cases.
Often (about 9% of cases) melanoma metastases on T2-weighted MRI were visualized as hyperintense lesions, while on T1-weighted MRI as isointense ones, which may be indicative of amelanotic melanoma.
Complications in the form of a hemorrhage in the tumor tissue in our material were often observed in particular in melanoma metastases (34% of patients were operated due to an increase in the size of metastatic lesions in the brain). It should be noted that the cause to perform neurosurgery was often extensive hemorrhages, not further growth of the solid part of the tumor.
It should be noted that not only melanoma metastases may have an increased MR signal on T1-weighted MRI but also primary tumors, for example, protein-containing lesions (craniopharyngioma, atypical epidermoids, colloid cysts in the third ventricle), lipomas and lipomatous meningiomas, dural osteomas, as well as abnormal non-tumor lesions (multiple sclerosis, hamartomas, neurofibromatosis type I, intracranial forms of infections, etc.). Tumors with hemorrhages and hematomas in the early subacute stage may have MR characteristics similar to those in melanomas. In addition, according to many authors, the diagnostic range must also include pigmented meningioma, meningeal melanocytoma, melanotic schwannoma, and melanoblastosis—neoplasms containing melanin in their structure (Tatagiba et al. 1992; Faro et al. 1996; Painter et al. 2000; Doglietto et al. 2012). The next chapter will describe the differential diagnosis of metastases.
The need to identify additional small multiple lesions determines the requirement for mandatory contrast enhancement of intracranial melanomas. Characteristics of accumulation of the contrast agent by melanoma metastases allowed to identify various patterns occurring as a result of the contrast agent accumulation. The main pattern of CA accumulation by melanoma metastases corresponded to the “homogeneous” type of accumulation and reached 76% in our material. However, in the presence of necrotic areas in the central part of the tumor site, CA accumulation occurred along the periphery—a homogeneous ring-shaped accumulation with clear contours—a “target.” Heterogeneous CA accumulation was also present but much less frequently. Perifocal edema was pronounced, which created, in turn, the picture of ring enhancement.
From our point of view, differences in the CA accumulation are due to, first of all, sizes, “age,” intensity of growth, metabolic activity of the tumor tissue, presence or absence of necrosis areas, hemorrhages, etc.
Thus, it can be concluded that the standard MRI methods (T1, T2, T1 weighted + contrast enhancement, T2-FLAIR) in case of metastatic brain lesions in melanoma not only provide information about the synoptic features of the tumor lesion(s) and their numbers and structure but also allow to suspect the source of metastases based on particular characteristics (a signal change on T2-weighted MRI).
Melanoma metastases on SWI (SWAN) MRI are included in our study as a separate group and were studied in detail using other additional MRI techniques. Thus, a decrease in MR signal in SWI (SWAN) images, similar to that in T2-weighted MRI, is quite typical and is caused by the characteristics of the melanoma cellular structure. Paramagnetic melanin itself causes local inhomogeneity of the magnetic field not only on SWI (SWAN) MRI but also on standard T2-weighted images, except for a small number of cases with the cystic structure of this lesion (Gaviani et al. 2006).
As noted above, growth of melanoma—one of the most malignant tumors—is often accompanied by hemorrhages into the tumor stroma. On SWI (SWAN) images, due to higher sensitivity of the pulse sequence to the field inhomogeneity, the overall low signal from the neoplasm covers some features of the internal structure of melanoma.
During an MR spectroscopic study in patients with suspected melanoma metastases, it was noted that shimming frequency in melanoma metastases in cases of its solid type exceeds 7 Hz and sometimes reaches up to 14 Hz. In metastatic tumors with other histological structure, this phenomenon is not detected. Most likely, this difference is related to the paramagnetic properties of melanin and hemorrhages associated with melanoma metastases. In other cases, on the background of a significant decrease in NAA and Cr peaks, there was a sharp increase in the lipid-lactate complex and a moderate increase in the choline peak. It should be noted that these results were obtained only in the presence of tumor necrosis. In our material, increases in the peaks of choline, lactate, and lipids in the central part of metastases were noted in only 22% of cases.
On DWI images, in patients with melanoma metastases in the brain, no characteristic features were identified that could allow to differentiate melanoma metastases from other focal lesions with necrotic changes in the tumor tissue. However, the use of DWI allowed to obtain additional information on melanoma metastases with the solid structure: melanoma metastases in contrast to lymphomas and gliomas are characterized by higher ADC values, as degradation in the metastasis stroma starts earlier. Unlike glial tumors, melanoma metastases have a lower signal on DWI MRI in the edema area, which is most likely due to the lack of infiltration of the brain substance by the tumor cells and more free movement of water molecules in the extracellular space. The ADC values obtained in the solid parts of melanoma metastases were 0.9 × 10−3 ± 0.05 × 10−3 s/mm2; in the lesions with necrosis signs, they were 2.18 × 10−3 ± 0.05 × 10−3 s/mm2; in the edema area, they were 1.92 × 10−3 ± 0.07 × 10−3 s/mm2; in the white matter on the unaffected contralateral side, they were 0.7 × 10−3 ± 0.3 × 10−3 s/mm2.
Melanoma metastases and primary tumor are very well visualized on both DWI MRI and PET with 18F-FDG. Since melanoma metastasizes to the soft tissues and bones, it is advisable to perform for diagnosis not scintigraphy, allowing to study only bone structures, but PET or MRI in order to investigate all tissues. 18F-FDG PET is also highly sensitive to amelanotic melanoma cells. In our material, all patients with melanoma metastases in the brain had foci with increased accumulation of the radiopharmaceutical even on the background of hemorrhagic inclusions. More than in a half (57%) of the studies conducted, the PET picture corresponded to multifocal neoplastic lesions of the internal organs and lymph nodes. A histological examination of the biopsy material obtained allowed to prove the presence of a malignant melanoma.
For a search of the primary tumor, a whole-body PET study can be performed at different stages of the diagnosis. An absolute indication for PET is the presence of multiple tumor lesions in the brain on MRI, absence of history of cancer, and negative results of other diagnostic methods. PET is often performed in patients with a quite long history of cancer that received surgical therapy in the past for some tumor. In such cases, additional tumor lesions and lymph nodes affected with metastases are often found. Based on the whole-body PET study, we found abnormal RP accumulation in 88.6% of patients with melanoma in our material (n = 44). It should be noted that a PET/CT study should include the upper and lower extremities, which often allows to identify additional lesions. Given the difficulties of using DWI MRI with inclusion of extremities in the region of interest, PET is the method of choice.
14.1 Clinical Cases
See Figs. 14.1, 14.2, 14.3, 14.4, 14.5, 14.6, 14.7, 14.8, 14.9, 14.10, 14.11, 14.12, 14.13, 14.14, 14.15, 14.16, 14.17, 14.18, 14.19, 14.20, 14.21, 14.22, 14.23, 14.24, 14.25, 14.26, 14.27, 14.28, 14.29, 14.30, 14.31, 14.32, 14.33, 14.34, 14.35, 14.36, 14.37, and 14.38.
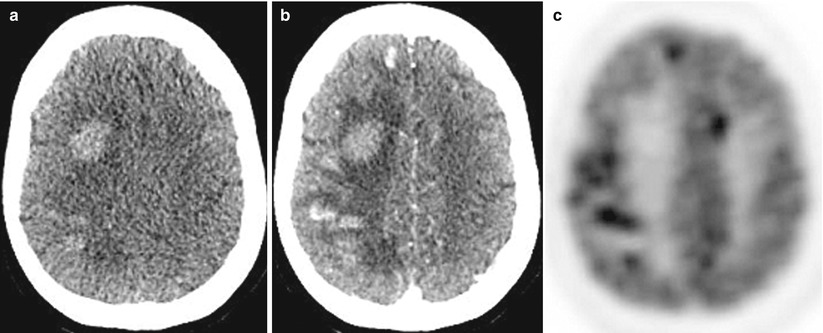
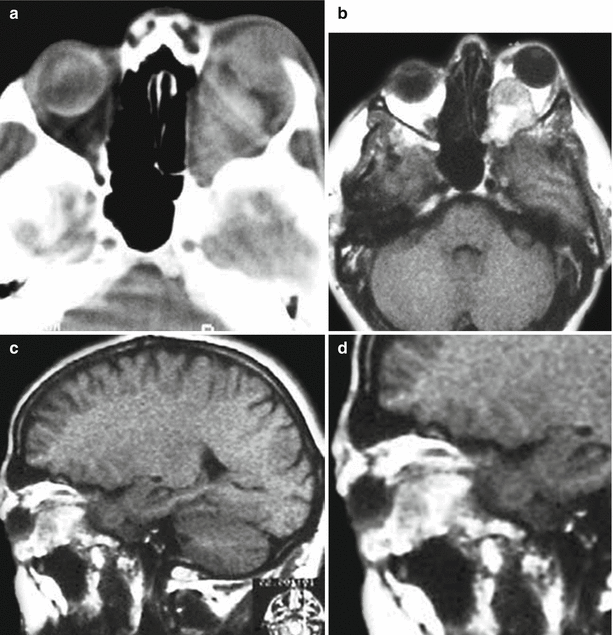
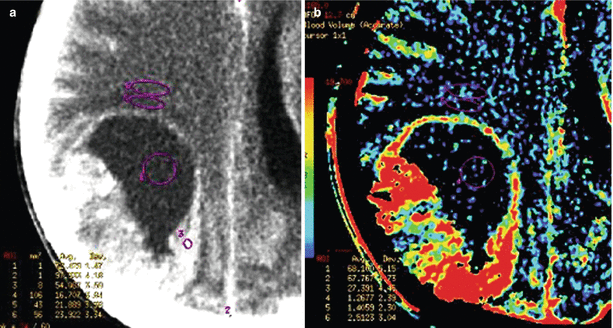
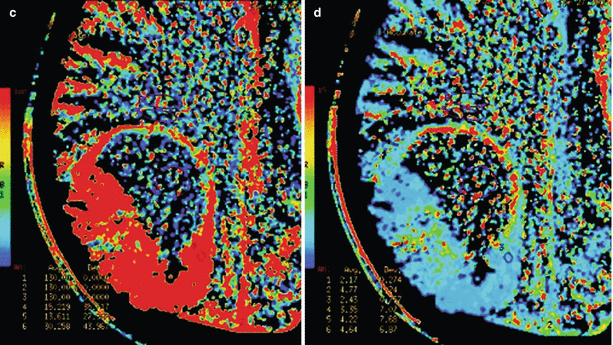
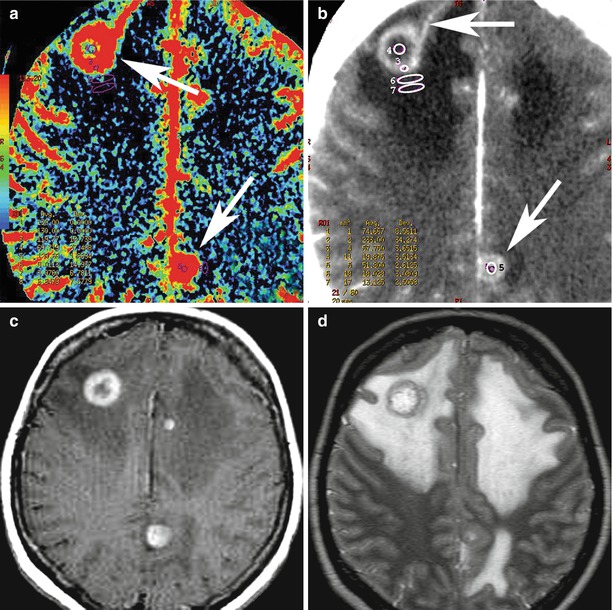
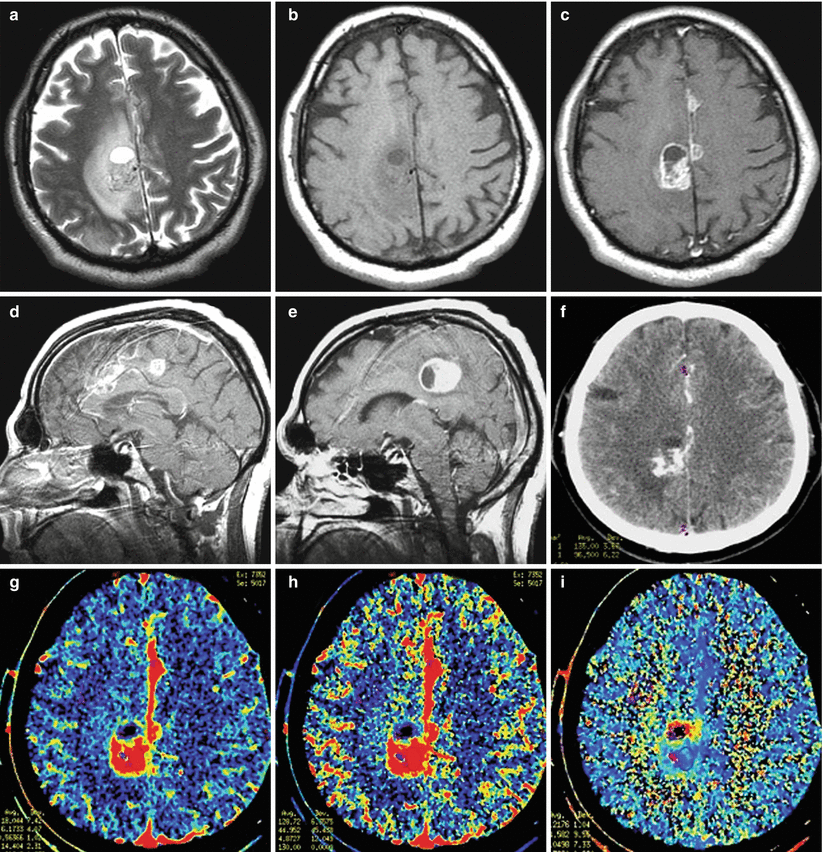

Fig. 14.1
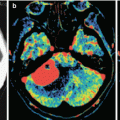
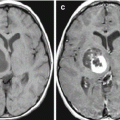
Multiple melanoma metastases in the brain. On a CT scan before the administration of the contrast agent (a), there are multiple areas with an increased density in the right frontal and parietal regions. After the administration of the contrast agent (b), multiple metastases are additionally identified in both frontal regions, with the largest lesion with no apparent CA accumulation—blood. On PET with 18F-FDG (c), lesions intensely accumulate RP, with the exception of the largest one, which is caused by a hemorrhage into its stroma

Fig. 14.2
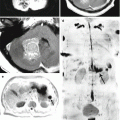
A melanoma metastasis in the left orbit. On a CT scan in axial projection (a), there is a weakly hyperdense space-occupying lesion that causes destruction of the medial wall of the left orbit and exophthalmos. T1-weighted MRI with contrast enhancement in the axial (b) and sagittal (c, d) projections allows to visualize a tumor located in the retrobulbar region, with moderate and uneven contrast enhancement. The optic nerve is displaced upward


Fig. 14.3
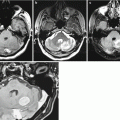
A melanoma metastasis in the brain. CT and CT perfusion maps. In the right parietal lobe, solid-cystic space-occupying lesion is identified, whose solid component (along the lateral contour) intensely accumulates CA (a). On CT perfusion maps of CBV (b), CBF (c), and MTT (d), typical for melanoma, high CBV and CBF values and low MTT values are observed in the solid structure of tumors

Fig. 14.4
Multiple metastases of melanoma. CBF map (a): melanoma metastases have elevated blood flow values (arrow). On CT with contrast enhancement (b), of note is a large artery approaching the front pole of the tumor (upper arrow) and an additional parasagittal tumor site (lower arrow). T1-weighted MRI with contrast enhancement (c) and T2-weighted MRI (d) allow to determine the true sizes of metastatic lesions and the severity of perifocal edema

Fig. 14.5




Multiple metastases of melanoma. There are multiple lesions along the falx on both sides in the frontal and parietal regions. The largest lesion in the right frontoparietal region on T2-weighted MRI (a) has a heterogeneously hyperintense signal, and the brain substance along the periphery of the lesion is edematous. On T1-weighted MRI (b), lesions are weakly differentiated (no hemorrhages). After CA administration—axial (c) and sagittal (d, e) projections—there is its pronounced accumulation in the tumor lesions, while the well-visualized cystic fragment is well visualized along the anterior pole of the largest metastasis. On CT (f), the lesion also intensely accumulates the contrast agent. On CT perfusion maps of CBV (g), CBF (h), and MTT (i), typical for melanoma, high CBV and CBF values and low MTT values are observed in the solid structure of tumors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree