WHO grade
Histological variants
% of total meningiomas
I—Benign
Transitional, fibroblastic, meningothelial, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, metaplastic
>90 %
II—Atypical
Clear cell, chordoid, atypical
5–7 %
III—Malignant
Anaplastic, papillary, rhabdoid
1–3 %
Comprising over 30 % of primary intracranial tumors, the incidence of meningiomas in the population is 4.5 per 100,000 person-years with females outnumbering males by 2:1 according to some series, but up to even 6:1 in others [6]. The incidence has also been shown to increase with age. Autopsy studies show that approximately 2.5 % of people have an incidental meningioma [7]. Anecdotal evidence has suggested several correlations with meningiomas. Cushing believed that head trauma was affiliated with tumorogenesis, but no definitive study has confirmed this despite multiple attempts to find a correlation [3, 8, 9]. Although several studies have attempted to link viral infection to the development of meningiomas, none have proved the causative effect [10]. There is also a link between breast cancer and meningioma that has been observed in women, but not in men. A study by Rao et al. showed that of 6,527 meningiomas and 153,599 breast cancers diagnosed in women and 2,327 meningiomas and 1,668 breast cancers diagnosed in men found in five cancer registries between 1995 and 2003, the rate of breast cancer in women already diagnosed with meningioma was 80 times expected, and the rate of meningioma in women already diagnosed with breast cancer was 58 times the expected rate. No such association could be found in the male patients [11].
Apart from female gender, there are two other major factors that do seem to lead to meningioma formation: radiation and chromosome 22 mutation. The incidence of meningioma has been found to be four times higher in patients who had tinea capitis treated with local radiation compared to control patients [12]. Another definitive correlation with meningiomas is deletions in the long arm of chromosome 22. Numerous patients with meningiomas have been found to have monosomy 22 and the gene thought responsible for meningioma formation has been localized to the same region as that of type 2 neurofibromatosis [13]. This may explain the high correlation between NF2 and the development of meningiomas.
The signs and symptoms associated with a meningioma depend primarily on its site of origin. The most common presentation is a new onset seizure, occurring approximately 30 % of the time [14]. These seizures are most often generalized, but can be partial or complex partial. Headache, mental status changes, hemiplegia, isolated cranial nerve deficits, and signs of increased intracranial pressure may also be seen in meningioma patients.
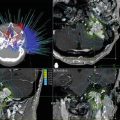
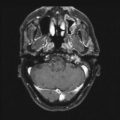
The radiographic diagnosis of meningioma is usually made with CT or MRI. Unenhanced CT shows meningiomas to be slightly hyperdense compared to normal brain, often with surrounding edema and variable mass effect; adding intravenous contrast results in homogeneous enhancement of the mass. MRI is the preferred method of diagnosis because of its superior soft tissue resolution. The tumor appears isointense to brain on an unenhanced T1-weighted image (T1WI), but hyperintense with surrounding white matter edema on a T2-weighted image (T2WI). The addition of gadolinium contrast reveals a uniformly enhancing mass with a broad-based connection to the surrounding dura. A dural tail is variably seen and represents growth of meningioma along the dura away from the main tumor. The significance of this finding is that if left untreated by either radiosurgery or surgery, since both are focal treatment modalities, the chance for recurrence increases [15]. These tumors are nearly always extra-axial, showing displacement of the cortex. Reactive sclerosis (hyperostosis) of the overlying bone can also be seen in some cases. Conventional catheter angiography, although increasingly supplanted by MR angiography and CT angiography, is often critical in the surgical planning because it can determine the blood supply to the tumor, degree of vascularity, patency of dural venous sinuses, and suitability for endovascular embolization. Meningiomas are primarily fed by branches from the external carotid artery, commonly the middle meningeal or occipital artery, although large tumors usually parasitize a significant blood supply from underlying small pial arteries.
Surgical Decision-Making and Techniques
Numerous factors must be addressed when evaluating a patient for surgery: age, location, size, symptoms, neurological deficit, tumor growth rate, proximity to critical structures, blood supply, venous drainage and sinus patency, previous treatments, and most importantly, patient expectations. A multidisciplinary approach with input from other disciplines such as ENT, radiation oncology, and radiology, when appropriate, is important.
The distinguishing advantage of surgery over radiosurgery is the ability to achieve a gross total resection. The incidence of tumor shrinkage following radiosurgery varies between 20 and 40 %, and the actual amount of decrease is fairly minor. Thus, overriding any other consideration is the fact that some patients will insist on having even a small benign tumor surgically removed. These patients should be offered an opportunity for surgical resection provided that the risks are acceptable. An optimal resection requires complete removal of the meningioma with all of its dural and bony attachments. In a classic paper by Simpson from 1957, he describes the recurrence rates for meningiomas following surgical resection [16]. A five tier grading system was devised to describe the extent of tumor removal, with grade I being the most complete and grade V being a simple decompression. Simpson noted that the recurrence rate following grade I resection was 9 %, grade II was 19 %, and grade III was 29 %. Later, a grade 0 was added to the Simpson scale [17, 18]. This added a 2 cm margin of normal appearing dura to a Simpson I resection. The recurrence rate of a patient undergoing a grade 0 resection of a benign meningioma is essentially zero. Clearly, the goal of surgery is to perform a grade 0 or a grade 1 resection; unfortunately, this may not be possible despite surgical expertise because the most important variable that influences extent of resection is tumor location.
Convexity Region
Convexity meningiomas are found on the dura over the hemispheres without extension into the dural sinuses or invasion of the skull base. They represent approximately 15 % of meningiomas and are the least likely to recur because they are nearly always amenable to a grade 0 or 1 resection. Intraoperative stereotactic guidance is helpful in localizing the projection of the tumor on the scalp. A generous scalp and craniotomy flap can then be tailored to allow for a 2 cm margin around the tumor. Preoperative embolization is less helpful than in other areas as the blood supply from dural feeders is easily interrupted by incising the dura circumferentially as the first step in tumor removal. Pial feeders are too small to be embolized and are divided during the course of tumor dissection off the brain. Large tumors may be adherent to the pia causing unavoidable cortical injury in the process of a complete removal. This superficial invasion does not constitute a sign of malignancy, but neurological deficit is possible and patients should be counseled appropriately. Surgery should be considered anytime a patient wants a cure of their convexity meningioma regardless of size and also recommended strongly in patients with a tumor larger than 3 cm. Radiosurgery is reserved for asymptomatic, small meningiomas, and the rare cases of recurrence following resection (see Case 21.1).
Parasagittal Region
Parasagittal meningiomas account for 20–30 % of meningiomas and describe a location without intervening brain tissue or dura between the tumor and the superior sagittal sinus (see Case 21.2). Conventional angiography or (CT or) MR venography is helpful in determining if the sinus is patent. When there is invasion of a venous sinus, a complete resection requires removal of the infiltrated sinus as well. This is ordinarily safe only with the anterior one-third segment of the sagittal sinus. The scalp and bone flap will need to cross the midline if the sagittal sinus is to be resected. Patients with middle and posterior third sagittal sinus involvement and a large tumor can benefit from a combined approach where the bulk of the tumor is removed, which relieves mass effect on the brain while leaving the residual tumor in the sinus to be treated by radiosurgery. Preoperative embolization may be useful in reducing blood loss during the debulking of large tumors.
Each case should be reviewed individually, and not all meningiomas need preoperative endovascular embolization. Factors such as the size and location of the tumor as well as the blood supply can influence the decision to embolize a tumor. Typically, there is no need for preoperative embolization of smaller size meningiomas at the convexity with dural feeding arteries since there is no significant benefit with regard to making the surgery safer for the patient or making the resection technically easier. However, large skull base meningiomas with deep feeding arteries that are large enough to cannulate in order to inject embolic material such as microspheres or liquid embolic agent may benefit from embolization by reducing blood loss and causing tumor necrosis, which will soften the tissue and lead to a technically easier removal of the devascularized tumor [19, 20]. Care must be taken to closely watch the patient after embolization because post-embolization swelling with significant mass effect on surrounding structures can occur. Some authors have suggested performing the preoperative embolization within 24 h prior to the operation to allow for some early necrosis and softening of the tumor tissue while minimizing the duration of time that the patient could potentially develop dangerous post-embolization edema. It is known that tumor vessels can recanalize if left for greater than 24–48 h after embolization, but this is more likely with some of the less durable liquid embolic agents that were used in the past. Current technique with microspheres and liquid embolic agents provide a much more durable occlusion and recanalization is not as much of a concern. Some have suggested waiting as long as a week in order to maximize the devascularizing necrosis effects after the embolization procedure [19, 20]. Embolization is usually not recommended as a sole treatment.
Sphenoid Wing and Anterior Skull Base
Sphenoid wing meningiomas are divided into lateral and medial depending on the site of origin. Lateral sphenoid wing meningiomas may invade the sphenoid bone, but complete resection is still possible provided the tumor is not big and the dura of the temporal or frontal floor not extensively invaded (see Case 21.3). Complete removal of a meningioma originating from the medial sphenoid wing is a difficult task for the surgeon because the tumor is likely involving the dura around the optic nerve, superior orbital fissure, and cavernous sinus (see Case 21.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree