CHAPTER 7 The main radiographic finding in osteoporosis is thinning of the cortex. This is best demonstrated in the second metacarpal at the middiaphysis (Figures 7-1 and 7-2). The normal metacarpal cortical thickening should be approximately one fourth to one third the thickness of the metacarpal (Figure 7-3). In osteoporosis this cortical thickness is decreased. The metacarpal cortex (and all bony cortices, for that matter) decreases normally with age and is less for women than for men of the same age. Several tables have been published that give the normal metacarpal cortical measurement, with age and sex adjustments to allow determination of normal. Unfortunately these tables determine only the mineralization of the peripheral skeleton and do not seem to correlate to whether or not spinal or hip fractures will occur. FIGURE 7-1 FIGURE 7-2 FIGURE 7-3 Measurement of the axial bone mineral content can be done by one of several methods that use computed tomography (CT) to assess the bone quantity in the spine. There is some debate over which method is superior and even whether or not knowing the bone mineral content is clinically helpful, because merely knowing the age and sex of the patient is fairly accurate for predicting the bone mass quantity. Nevertheless, it is generally agreed that if a quantitative CT (QCT) measurement shows bone mass to be 2 standard deviations below normal, that person is at high risk for spinal and hip fractures. Bone densitometry is widely used to identify patients, especially postmenopausal women, who are osteoporotic so that treatment can be instituted. Bisphosphonates have been widely used for treating osteoporosis with excellent results for the most part. A complication of long-term bisphosphonate therapy is fracture of the proximal femur.1 These fractures occur on the lateral aspect of the proximal femoral diaphysis and are often bilateral (Figure 7-4). If not treated, they can go on to complete fractures. FIGURE 7-4 Exercise and proper diet (whatever that is) seem to help delay the onset of primary osteoporosis as much as anything. Calcium additives alone have not been shown to reverse the process of primary osteoporosis.2 Because we cannot accurately give the causes of osteoporosis by looking at a radiograph and cannot even differentiate it from osteomalacia, it is a topic that is frustrating for many radiologists to deal with. Most of us would rather comment on something we can give a diagnosis on or at least a short differential. In general, when decreased bone mass is present on a radiograph, the odds are that osteoporosis is present. However, because the disease process could just as easily be osteomalacia, it is recommended that the term osteopenia be used. This is a generic term that includes both osteoporosis and osteomalacia. When used, it also implies that the observer knows he or she cannot separate the two entities and is an educated person. A type of osteoporosis that can be seen in a patient of any age is disuse osteoporosis. It results from immobilization from any cause, most commonly after treatment of a fracture. The radiographic appearance of disuse osteoporosis is different from primary osteoporosis in that it occurs somewhat more rapidly and gives the bone a patchy or even a permeative appearance (Figure 7-5). This is from the osteocytic resorption in the cortex, causing intracortical holes. If allowed to continue with disuse, the bone would resemble any bone with marked osteoporosis (i.e., severe cortical thinning). FIGURE 7-5 Occasionally aggressive osteoporosis from disuse can mimic a permeative lesion, such as a Ewing’s sarcoma or multiple myeloma, because of the severe cortical patchy or permeative pattern that projects over the medullary space and resembles a medullary permeative process (Figure 7-6). The way to differentiate a true intramedullary permeative process from an intracortical process such as osteoporosis is to observe the cortex and see if it is solid or riddled with holes (Figure 7-7). If it is solid, you can assume the permeative process is emanating from the medullary space (Figure 7-8); if it has multiple small holes, you have to assume the permeative pattern is from the cortical process. I call a permeative appearance that is secondary to cortical holes a pseudopermeative process to distinguish it from a true permeative process. FIGURE 7-6 FIGURE 7-7 FIGURE 7-8 Another cause for a pseudopermeative process is a hemangioma. It can cause cortical holes in two ways: from focal increased blood flow or hyperemia, causing focal osteoporosis, or by the blood vessels themselves tunneling through the cortex (Figure 7-9). I have seen more than one hemangioma operated on inadvertently because the lesion was thought to be a Ewing’s sarcoma—they bleed a lot. FIGURE 7-9 Radiotherapy can cause cortical holes in bone and mimic a permeative pattern (Figure 7-10). These holes are often large and would not be confused with a true permeative process, but they can be small and cause confusion. FIGURE 7-10 If a permeative pattern is seen in bone, the differential is usually an aggressive process such as Ewing’s sarcoma, infection, or eosinophilic granuloma in a young person or multiple myeloma, metastatic carcinomatosis, or primary lymphoma of bone in an older patient. If, however, the permeative pattern is seen to be a result of cortical holes (i.e., a pseudopermeative pattern), the differential is considerably kinder: aggressive osteoporosis, hemangioma, or radiation changes. This differential does not arise often but is very useful when it does come up.3
Metabolic bone disease
Osteoporosis
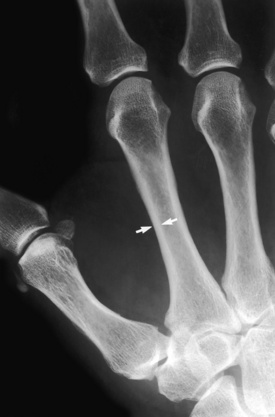
 Mild osteoporosis. Mild cortical narrowing (arrows) at the mid-second metacarpal is noted in this patient with renal osteodystrophy. Compare this cortical width with that of the normal width in Figure 7-3.
Mild osteoporosis. Mild cortical narrowing (arrows) at the mid-second metacarpal is noted in this patient with renal osteodystrophy. Compare this cortical width with that of the normal width in Figure 7-3.
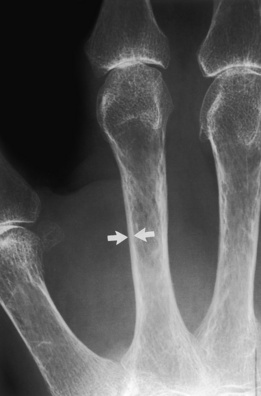
 Marked osteoporosis. Marked cortical narrowing (arrows) of the mid-second metacarpal cortex is present in this patient with severe osteoporosis. Also note the intracortical tunneling, which occurs with more rapid forms of osteoporosis.
Marked osteoporosis. Marked cortical narrowing (arrows) of the mid-second metacarpal cortex is present in this patient with severe osteoporosis. Also note the intracortical tunneling, which occurs with more rapid forms of osteoporosis.
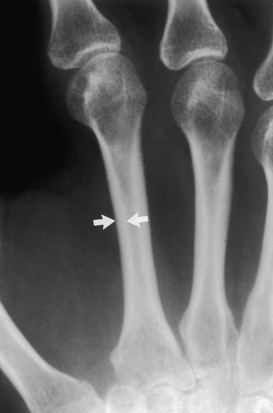
 Normal mineralization. Note the cortical width (arrows) of the mid-second metacarpal in this patient with normal mineralization. The width of the cortex is easily greater than one third of the total width of the metacarpal.
Normal mineralization. Note the cortical width (arrows) of the mid-second metacarpal in this patient with normal mineralization. The width of the cortex is easily greater than one third of the total width of the metacarpal.
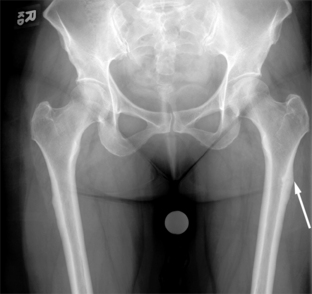
 Femoral fracture secondary to bisphosphonate therapy. This postmenopausal woman had been taking bisphosphonates for many years and sought treatment for left thigh pain. Focal cortical thickening (arrow) is characteristic for the appearance of femur fractures related to bisphosphonate therapy. These can go on to a complete fracture if not protected or treated.
Femoral fracture secondary to bisphosphonate therapy. This postmenopausal woman had been taking bisphosphonates for many years and sought treatment for left thigh pain. Focal cortical thickening (arrow) is characteristic for the appearance of femur fractures related to bisphosphonate therapy. These can go on to a complete fracture if not protected or treated.
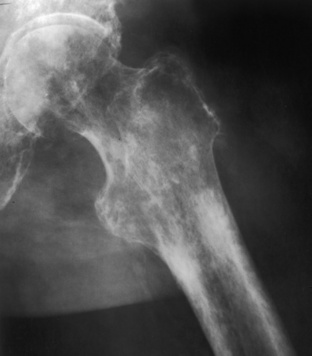
 Aggressive osteoporosis. A diffuse permeative pattern throughout the proximal femur is noted in this patient, who has recently had an amputation. Note that the cortices are riddled with holes, which would indicate that this is not a true intramedullary process but an intracortical process. This is distinctive for aggressive osteoporosis and causes a pseudopermeative pattern that can be mistaken for a more sinister process.
Aggressive osteoporosis. A diffuse permeative pattern throughout the proximal femur is noted in this patient, who has recently had an amputation. Note that the cortices are riddled with holes, which would indicate that this is not a true intramedullary process but an intracortical process. This is distinctive for aggressive osteoporosis and causes a pseudopermeative pattern that can be mistaken for a more sinister process.
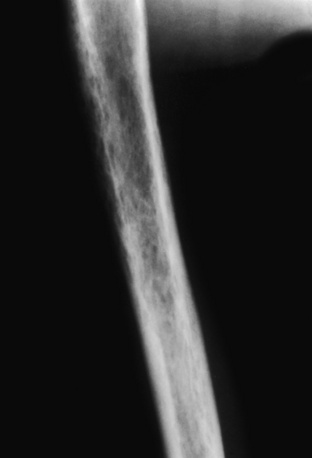
 Cortical holes in osteoporosis. This patient suffered a stroke and has aggressive osteoporosis secondary to disuse. What appears to be a diffuse permeative pattern throughout the humerus is noted, on closer inspection, to be cortical holes, which in this case resulted from the aggressive osteoporosis. This type of pattern, unfortunately, often leads to a biopsy to rule out multiple myeloma or other round cell tumors.
Cortical holes in osteoporosis. This patient suffered a stroke and has aggressive osteoporosis secondary to disuse. What appears to be a diffuse permeative pattern throughout the humerus is noted, on closer inspection, to be cortical holes, which in this case resulted from the aggressive osteoporosis. This type of pattern, unfortunately, often leads to a biopsy to rule out multiple myeloma or other round cell tumors.
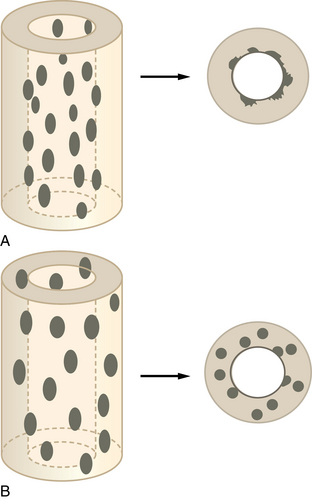
 Schematic of cortical holes. A, This schematic of a permeative lesion shows how only the endosteum is affected with the bulk of the cortex spared. B, A pseudopermeative lesion affects the entire cortex. Both processes will look permeative on a plain film; however, the true permeative process will have an uninvolved cortex.
Schematic of cortical holes. A, This schematic of a permeative lesion shows how only the endosteum is affected with the bulk of the cortex spared. B, A pseudopermeative lesion affects the entire cortex. Both processes will look permeative on a plain film; however, the true permeative process will have an uninvolved cortex.
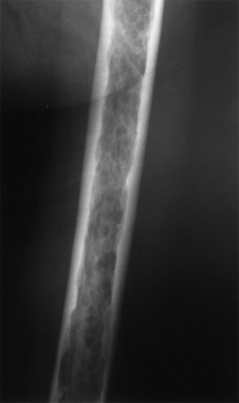
 Permeative appearance in myeloma. This patient has multiple myeloma, which has caused a diffuse permeative pattern throughout his femur. Note the solid appearance of the cortex, although there is endosteal scalloping. This is a true permeative process.
Permeative appearance in myeloma. This patient has multiple myeloma, which has caused a diffuse permeative pattern throughout his femur. Note the solid appearance of the cortex, although there is endosteal scalloping. This is a true permeative process.
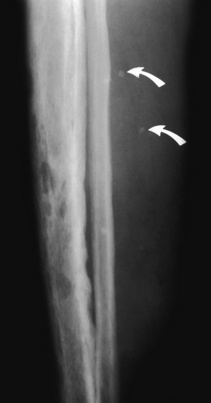
 Hemangioma. Cortical holes of varying sizes are seen throughout the tibia in this patient with a large hemangioma of the tibia. Also note the phleboliths present in the soft tissues (arrows). It would be unlikely for osteoporosis to cause cortical holes of this size.
Hemangioma. Cortical holes of varying sizes are seen throughout the tibia in this patient with a large hemangioma of the tibia. Also note the phleboliths present in the soft tissues (arrows). It would be unlikely for osteoporosis to cause cortical holes of this size.
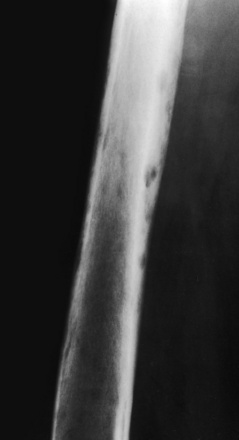
 Osteonecrosis secondary to radiation. Radiation to the femur for a soft tissue sarcoma some years before this plain film was taken has caused large cortical holes throughout the femur. It is not likely that cortical holes of this size would be caused by osteoporosis, but they could be caused by a hemangioma.
Osteonecrosis secondary to radiation. Radiation to the femur for a soft tissue sarcoma some years before this plain film was taken has caused large cortical holes throughout the femur. It is not likely that cortical holes of this size would be caused by osteoporosis, but they could be caused by a hemangioma.
Radiology Key
Fastest Radiology Insight Engine



