Benjamin D. Levine, MD
Kambiz Motamedi, MD
Leanne L. Seeger, MD
INTRODUCTION
Metabolic diseases of bone include those skeletal disorders related to abnormalities that involve the anabolic and catabolic biochemical reactions in the body. Such disorders include, but are not limited to, endocrine dysfunctions, Paget disease, and osteoporosis. The focus of this chapter is to discuss several of the metabolic bone diseases with emphasis on their imaging features and differential diagnosis.
HYPERPARATHYROIDISM
Parathyroid hormone (PTH) is intimately involved in calcium homeostasis. PTH not only regulates the release of calcium from bone through its combined effects on osteoblasts and osteoclasts but also stimulates bone remodeling. Hyperparathyroid states, reflecting excess circulating levels of PTH, are traditionally divided into three types: primary, secondary, and tertiary. Primary hyperparathyroidism is characterized by the excessive production of PTH by one or more of the parathyroid glands, most commonly a solitary hyperfunctioning adenoma (up to 80% of cases). Primary hyperparathyroidism may rarely be caused by a parathyroid carcinoma (<1%).1 Other causes of primary hyperparathyroidism include diffuse hyperplasia of the parathyroid gland and multiple hyperfunctioning adenomas. Secondary hyperparathyroidism results from prolonged hypocalcemia, as in cases of chronic renal failure or intestinal malabsorption. Tertiary hyperparathyroidism may result from long-standing secondary hyperparathyroidism, with the development of autonomous activity due to parathyroid hyperplasia and loss of the glands’ response to levels of serum calcium.
Hyperparathyroidism is characterized by an increased ratio of osteoclasts to osteoblasts. Because osteoclasts do not express a PTH receptor, the skeletal effects of hyperparathyroidism are mediated through the osteoblasts that communicate in some way with the osteoclasts.2 Skeletal manifestations of hyperparathyroidism are varied, and include osteitis fibrosa (vascular fibrous tissue replacement), osteitis fibrosa cystica (coalescence into cysts), cysts, brown tumors, thinned cortices, distorted trabeculae, infarction, and fracture.
 Imaging Findings
Imaging Findings
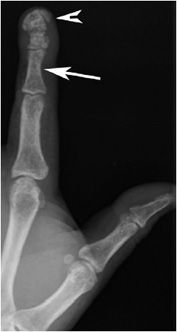
The most common radiographic bone abnormality in patients with hyperparathyroidism, regardless of cause, is bone resorption. The most characteristic type of bone resorption associated with hyperparathyroidism is subperiosteal (Figure 8-1), although intracortical, endosteal, subchondral, subphyseal, subligamentous, and subtendinous resorption may be noted. Other bony findings may include brown tumors and osteosclerosis. There is an association of primary hyperparathyroidism with calcium pyrophosphate dihydrate crystal deposition disease (CPPD), and rarely with gout.3
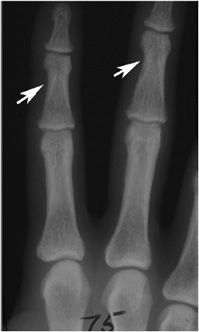
Figure 8-1. Hyperparathyroidism. PA view of the hand demonstrates subperiosteal resorption (arrows) in a characteristic location along the radial aspect of the index and long middle phalanges.
Subperiosteal resorption of cortical bone can be found in many locations, including the medial aspects of the proximal tibia, humerus, and femur. Acroosteolysis of the distal phalanges is characterized by subperiosteal resorption of the cortex of the terminal tuft. Subperiosteal resorption is most frequently detected along the radial aspects of the middle phalanges of the index and long fingers. For unknown reasons, the ulnar aspects of these bones are less affected. The earliest change is a “lace-like” appearance of the cortical surface that progresses to irregular spiculation and ultimately to loss of the entire cortex.4
The imaging findings of subperiosteal resorption can also be found at the margins of joints, most commonly the acromioclavicular, sternoclavicular, and sacroiliac joints. In more advanced cases, where complete or near complete resorption of the bony cortex has occurred, the foci of resorption can mimic the erosions of rheumatoid arthritis and the pressure atrophy of bone that may simulate scleroderma. The presence of subperiosteal resorption along the shafts of the phalanges is a useful discriminating tool.
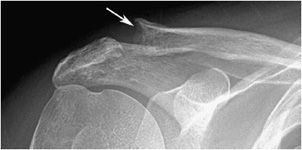
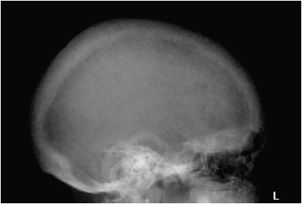
Bone resorption can also occur at other sites, where it is considerably less specific. Intracortical resorption, or tunneling, appears radiographically as prominent longitudinal, linear striations. This is usually best seen along the cortex of the long finger metacarpal and may also be found in rapidly occurring osteoporosis such as that accompanying disuse. Endosteal resorption demonstrates scalloping of the inner cortical margin. Findings of subchondral resorption are found primarily in the axial skeleton, including the sacroiliac joints, acromioclavicular joints (Figure 8-2), and symphysis pubis (Figure 8-3). The radiographic findings are bilaterally symmetrical and include resorption underlying bone.5 Cancellous (trabecular) bone resorption can occur throughout the skeleton in severe hyperparathyroidism, and involvement of the cranium results in a mottled “salt and pepper” pattern (Figure 8-4). Subligamentous and subtendinous resorption in hyperparathyroidism is most frequent along the femoral trochanters, ischial tuberosities (Figure 8-5), and inferior aspect of the distal clavicle.6
Figure 8-2. Hyperparathyroidism. AP view of the shoulder demonstrates subchondral resorption of the distal clavicle (arrow).
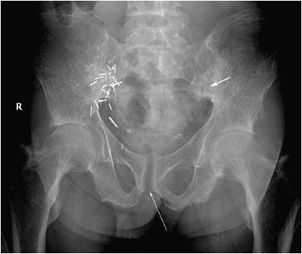
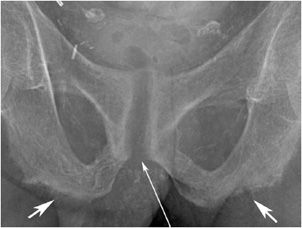
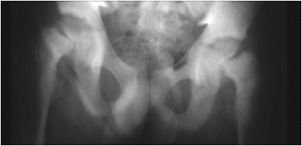
Figure 8-3. Hyperparathyroidism. AP view of the pelvis shows bone resorption about the symphysis pubis (long arrow) and sacroiliac joints (short arrow). Also note the diffuse osteopenia.
Figure 8-4. Hyperparathyroidism. Lateral view of the skull demonstrates trabecular bone resorption resulting in the “salt and pepper” appearance of the skull.
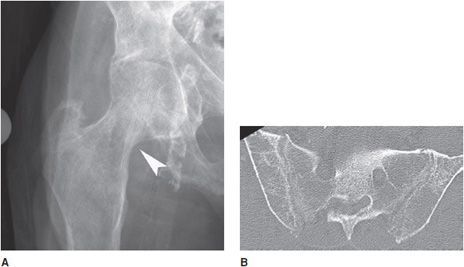
Figure 8-5. Hyperparathyroidism. Subligamentous and subtendinous bone resorption in the ischial tuberosities (short thick arrows). Note the resorption of the pubic symphysis (long thin arrow).
Brown tumors are associated with secondary hyperparathyroidism, although the incidence is greater in primary hyperparathyroidism. Those associated with secondary hyperparathyroidism are on the rise given the growing incidence of chronic renal disease in older populations undergoing dialysis. Pathologically, these bone lesions represent replacement of the normal osseous architecture by focal cyst-like accumulations of fibrous tissue and multinucleated giant cells following microfractures in areas of bone resorption. The “brown” color comes from the increased vascularity and hemosiderin deposition that is associated with these lesions.2
Brown tumors may be solitary or multifocal; can occur anywhere in the axial or appendicular skeleton; tend to be cortically based; and are most commonly seen in the pelvis, ribs, clavicles, and facial bones (Figures 8-6 and 8-7). radiographically, these lesions usually have a bubbly, cystic appearance and may be expansile. With excision of the parathyroid adenoma these lesions may become sclerotic, before eventually involuting completely (Figure 8-8). The presence of other radiographic findings of hyperparathyroidism is critical when differentiating brown tumors from other bone lesions.
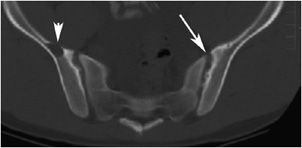
Figure 8-6. Hyperparathyroidism. Axial CT image of the pelvis shows a lytic lesion in the right iliac bone consistent with a brown tumor (arrowhead). Note the subchondral resorption about the sacroiliac joints (arrow).
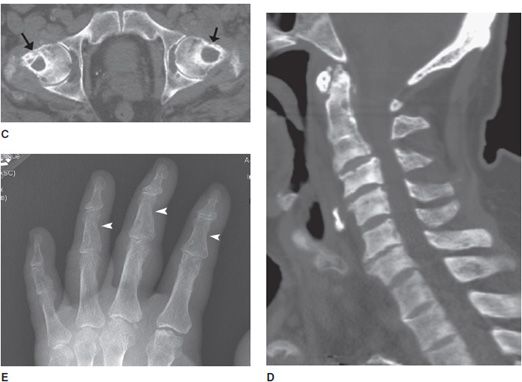
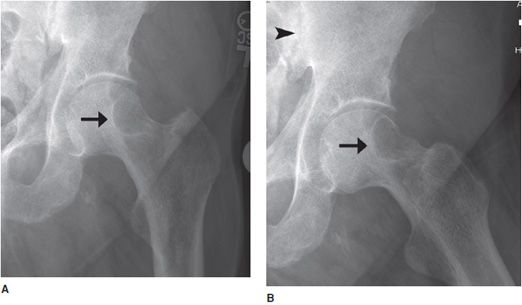
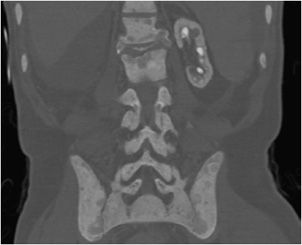
Figure 8-7. Brown tumor. Patient is a 65-year-old male with renal osteodystrophy. AP (A) and lateral (B) radiographs of left femur show lytic lesion of the femoral head and neck region that is a brown tumor (arrow). Note, erosion of the sacroiliac joint (arrowhead). (C) Brown tumor of the femoral heads and necks. CT of the pelvis shows lytic lesions of the femoral head and neck that are brown tumors (arrows). (D) Sagittal CT of the cervical spine shows sclerosis of the bones due to renal osteodystrophy. (E) Renal osteodystrophy and secondary hyperparathyroidism shows subperiosteal resorption in radial aspect of phalanges (arrowheads).
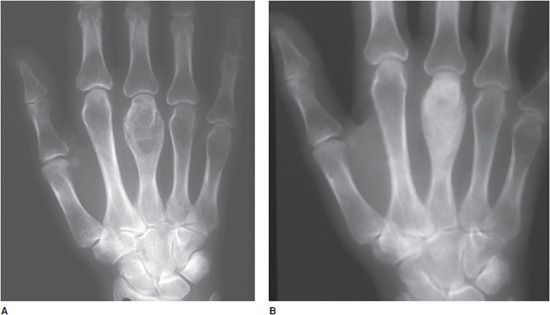
Figure 8-8. Hyperparathyroidism. (A) PA view of the hand demonstrates the lytic expansile brown tumor in the third metacarpal. (B) After parathyroidectomy, note the remineralization of the brown tumor.
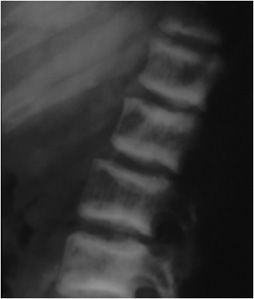
Since PTH primarily acts upon osteoblasts, there may be associated osteosclerosis. The sclerotic changes are seen more frequently in secondary hyperparathyroidism. While there are rare reports of diffuse bone sclerosis, the sclerotic changes tend to be focal. In the spine, sclerotic bands of trabecular bone subjacent to the superior and inferior endplates of the vertebral bodies have the appearance of a “rugger jersey” because of their similarity to the striped shirts worn by rugby players (Figure 8-9). Sclerotic changes may also be found within the skull and metaphyses of the long bones.
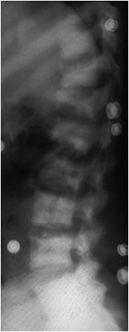
Figure 8-9. Hyperparathyroidism. Lateral view of the lumbar spine demonstrates condensation of bone along the superior and inferior endplates consistent with the so-called “rugger jersey” spine.
 Differential Diagnosis
Differential Diagnosis
Subperiosteal bone resorption is the radiographic hallmark of hyperparathyroidism and is characteristically located along the radial aspect of the index and long finger middle phalanges. Subperiosteal erosion may mimic resorption and may be caused by infection, tumor, and sarcoid. Other types of bone resorption seen in hyperparathyroidism such as endosteal resorption can also be seen in multiple myeloma and rapidly occurring osteoporosis.
Brown tumors, typically bubbly or cystic in appearance, can mimic primary bone tumors including aneurysmal bone cyst, giant cell tumor, fibrous dysplasia, and metastasis. Differentiation from metastasis may become more difficult with the presence of more than one brown tumor, in which the identification of additional features of hyperparathyroidism is critical.
The “rugger jersey” sclerotic bands subjacent to the vertebral body endplates in hyperparathyroidism must be differentiated from the “picture frame” vertebrae of Paget disease (Figure 8-10), where sclerotic changes are found around the perimeter of the involved vertebral body rather than just at the endplates. The bone in Paget disease is also enlarged with thickened trabeculae. Other differential considerations include osteopetrosis (“sandwich vertebra”) and corticosteroid induced osteoporosis, with the latter associated with concave appearance of the endplates.1
Figure 8-10. Paget disease. Lateral view of the spine demonstrates sclerosis around the entire vertebral body that resembles a picture frame (arrow). The vertebral body is also enlarged with thickened trabeculae.
Finally, CPPD crystal deposition that may be associated with primary hyperparathyroidism is identical to that found in idiopathic CPPD.4
PEARLS
RENAL OSTEODYSTROPHY
Renal osteodystrophy refers to bone disease seen in patients with chronic renal failure. Osseous abnormalities of renal osteodystrophy and chronic renal disease include those related to hyperparathyroidism, rickets, and osteomalacia, osteoporosis, fractures, and calcifications in the soft tissues and the media of arterial walls. Also included are the many musculoskeletal manifestations of dialysis in patients with end-stage renal disease.
 Imaging Findings
Imaging Findings
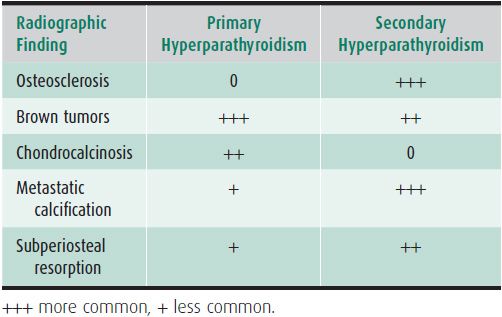
Radiographic abnormalities associated with hyperparathyroidism may also become manifest in renal osteodystrophy. These include bone resorption, brown tumors, osteosclerosis (Figure 8-11), and the so-called “metastatic calcification” of soft tissue and arterial walls. Certain findings of hyperparathyroidism occur with greater frequency in the secondary form associated with chronic glomerular renal disease (Table 8-1). Formerly, brown tumors were less frequently seen in secondary hyperparathyroidism than in the primary form. Osteosclerosis, such as the “rugger jersey” spine, is more common in the secondary form.1 Chondrocalcinosis is not associated with secondary hyperparathyroidism.
Figure 8-11. Renal osteodystrophy. Coronal CT image demonstrates extensive osteosclerosis of the spine and pelvis. Note the small, calcified left kidney and absent right kidney from prior nephrectomy in this patient with chronic renal disease.
Table 8-1. Primary Versus Secondary Hyperparathyroidism
Osteomalacia is a finding of chronic renal disease, and in children is termed rickets. It leads to excessive production of poorly mineralized osteoid. The cause of osteomalacia in chronic renal disease is complex but likely reflects the diseased kidney’s inability to effectively convert vitamin D into its active metabolite, 1,25-dihydroxycholecalciferol. In addition, patients with chronic renal tubular failure have malabsorption of calcium from the GI tract, which also contributes to bone disease.
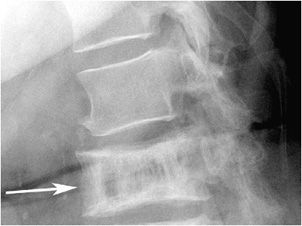
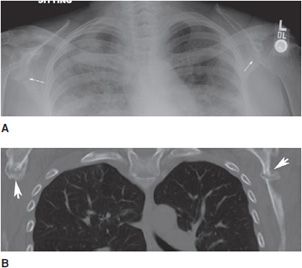
Typical radiographic features of osteomalacia include osteopenia, Looser zones, and bony abnormalities related to softening. Looser zones, which are also known as pseudofractures, represent insufficiency fractures that appear as transverse radiolucent bands. These are oriented perpendicular to the cortex, tend to be symmetric bilaterally, and may have sclerotic margins. Commonly involved locations include the pubic rami, ilia, axillary margin of the scapula, femoral neck, and ribs (Figure 8-12).
Figure 8-12. Looser zones. AP chest radiograph (A) shows typical pseudofractures of osteomalacia at the axillary margin of both scapulae (arrows). These tend to be bilaterally symmetric, are perpendicular to the cortex, and have sclerotic margins. Coronal CT image (B) again demonstrates the Looser zones of osteomalacia (arrowheads).
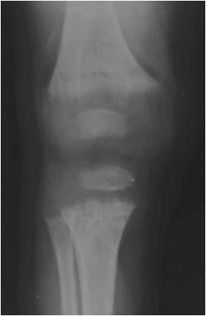
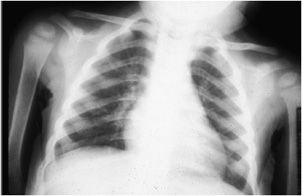
In children, the findings of rickets are related to nonmineralized osteoid in and around the growth plates or physes. This is due to a lack of mineralization of the provisional zone of calcification of cartilage layers at the growth plate. Early radiographic findings include apparent widening of the growth plates. With more severe changes, the long bone metaphyses become flared and frayed (Figure 8-13). Common sites of involvement include the knee on the distal femur or proximal tibia, the distal radius and ulna, and the costochondral junctions (Figure 8-14), where the foci of bone overgrowth represent “rachitic rosary.”
Figure 8-13. Rickets. AP radiograph of the knee shows physeal widening due to absence of mineralization of provisional zone of calcification due to rickets. Note cupping and brushing of the metaphysis with flared and frayed metaphyses characteristic of rickets.
Figure 8-14. Rickets. AP radiograph of the chest in a child shows widening of the growth plates of the humeri and prominence of the anterior costochondral junctions of the ribs (“rachitic rosary”).
Soft tissue and vascular calcifications are yet another manifestation of renal osteodystrophy, and represent the metastatic calcification of secondary hyperparathyroidism (Figure 8-15). Vascular calcifications can occur in the media of arteries, including the coronary arteries and the small peripheral arteries. Soft tissue calcifications may be seen around the ocular tissues, intra-abdominal viscera, mucosa of stomach, lungs, and the periarticular soft tissues. Tumoral calcinosis refers to the large heterogeneous and lobular mass-like calcifications adjacent to joints. The regions of the hip and elbow are the most common sites of involvement (Figure 8-16).
Figure 8-15. Renal osteodystrophy. PA view of the hand shows metastatic calcification about the index finger distally (arrowhead). Note the subperiosteal resorption along the radial aspect of the middle phalanx indicating secondary hyperparathyroidism (arrow).
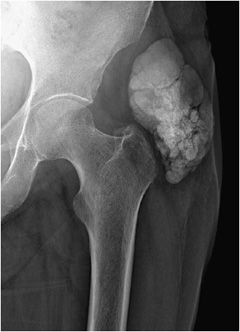
Figure 8-16. Tumoral calcinosis. AP radiograph of the hip demonstrates large lobular mass-like calcification in the soft tissues adjacent to the joint in this patient with secondary hyperparathyroidism.
When patients with end-stage renal disease are treated with hemodialysis, many of the musculoskeletal findings resolve, while others progress and new ones emerge. Although soft tissue calcifications may regress, they more commonly progress. Progression of findings of bone density loss and osteomalacia may reflect the use of aluminum-containing binders. Further weakening of the bones predisposes patients to fractures. Hemodialysis is thought to cause immunosuppression. Many patients are also taking corticosteroids, leading to other potential complications including osteomyelitis, septic arthritis, and avascular necrosis.
Patients on long-term hemodialysis, usually more than 10 years, may develop an idiopathic destructive spondyloarthropathy form of amyloidosis, thought to be secondary to the accumulation of excessive circulating β-2 microglobulin. Radiographic findings include loss of the disc space, end-plate, and subchondral erosions of the vertebral body as well as bony productive change.7 These findings are most commonly seen in the cervical spine and with lesser frequency in the lumbar and thoracic spine.
Hemodialysis-related amyloid may lead to arthralgias and arthropathies, particularly about the shoulder, but also around the hip and wrist (Figures 8-17–8-19). Radiographs demonstrate variable sized juxta-articular lytic lesions, which may be surrounded by fine sclerosis. The findings are often bilateral and may lead to pathologic fractures. The typical juxta-articular location helps to distinguish these focal amyloid deposits from multiple myeloma and metastatic disease, which may have a similar appearance.8
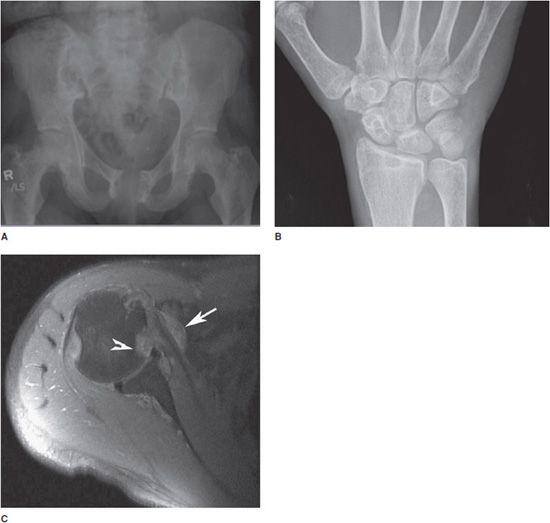
Figure 8-17. Dialysis-related amyloidosis. (A) AP pelvis radiograph demonstrates bone resorption about the sacroiliac joints and pubic symphysis in patient on long-term dialysis. (B) PA radiograph of the hand shows lytic lesions of the distal radius and carpal bones. (C) Axial MRI image demonstrates intermediate signal foci of amyloid deposition about the subscapularis tendon (arrow) and glenohumeral joint, with lytic lesions filled with amyloid in the humeral head (arrowhead).
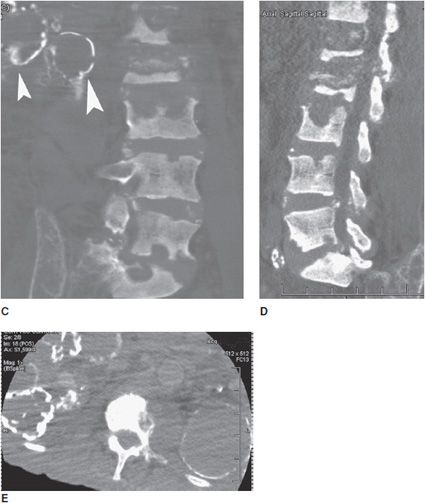
Figure 8-18. Renal osteodystrophy. Patient is a 74-year-old male with polycystic kidney disease. (A) AP radiograph of the right hip shows narrowing of the femoral neck due to subperiosteal resorption (arrowhead). (B) Axial CT of the pelvis shows subchondral bone resorption in sacroiliac joints. (C) Coronal CT reformat of lumbar spine shows multiple bone erosions of vertebral endplates of the lumbar spine. Note calcified cystic changes of kidney on right side (arrowheads). (D) Sagittal CT reformat of lumbar spine shows multiple bone erosions of vertebral endplates of lumbar spine. (E) Axial CT of the lumbar spine shows calcified cystic changes in both kidneys.
Figure 8-19. Amyloidosis. Lateral radiograph of lumbar spine of patient with renal osteotrophy on dialysis. Note erosions of the vertebral endplates due to amyloidosis. Also note generalized mixed osteoporosis and osteosclerosis due to renal osteodystrophy and presence of “rugger jersey” spine.
 Differential Diagnosis
Differential Diagnosis
Renal osteodystrophy is a term encompassing a wide variety of radiographic and clinical abnormalities. The diagnosis should not be made unless the appropriate clinical and radiological criteria are satisfied. Specifically, features of secondary hyperparathyroidism should be distinguished from those of primary hyperparathyroidism. In secondary hyperparathyroidism, there is an increased frequency of vascular and soft tissue calcification and osteosclerosis. Vascular calcifications may be seen in patients with long-standing atherosclerotic disease, diabetes mellitus, and primary hyperparathyroidism. Periarticular calcification can also be seen in various collagen vascular diseases, hypervitaminosis D, milk-alkali syndrome, and idiopathic tumoral calcinosis. Soft tissue calcification of renal osteodystrophy is usually associated with other radiographic features of the condition.
There are a myriad of causes of osteoporosis, which may be indistinguishable radiographically from osteomalacia. Primary forms are related to normal aging. Secondary forms may be related to the use of medications such as corticosteroids and hormonal disturbances such as pregnancy and diseases of the thyroid gland. Rachitic changes of tubular renal osteodystrophy are nearly identical to those due to dietary deficiencies. Slipped epiphyses may be more common in rickets-associated chronic renal disease, and are overall associated with chronic renal disease in about 10% of children (Figure 8-20).1
Figure 8-20. Slipped capital femoral epiphysis. AP radiograph of pelvis shows bilateral slipped femoral epiphyses and generalized osteosclerosis in this child with renal osteodystrophy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree