Jean Noel Buy1 and Michel Ghossain2
(1)
Service Radiologie, Hopital Hotel-Dieu, Paris, France
(2)
Department of Radiology, Hotel Dieu de France, Beirut, Lebanon
12.1 General Features
12.2 Metastatic Tumors
12.2.1 Breast
12.2.2 Female Genital Tract
12.2.3 GI Tract
12.2.4 Urinary Tract
12.2.5 Melanoma
12.2.7 Extragenital Sarcomas
Abstract
When an ovarian mass suspicious for malignancy is identified at an imaging examination, primary ovarian cancer is generally the main concern [1]. However, malignant tumors of the ovary are metastatic in 5–7 % of cases [2]. While metastases are uncommon in daily practice, metastatic neoplasms may be misdiagnosed as primary ovarian neoplasms [3, 4], potentially leading to inappropriate management [1].
12.1 General Features
When an ovarian mass suspicious for malignancy is identified at an imaging examination, primary ovarian cancer is generally the main concern [1]. However, malignant tumors of the ovary are metastatic in 5–7% of cases [2]. While metastases are uncommon in daily practice, metastatic neoplasms may be misdiagnosed as primary ovarian neoplasms [3, 4], potentially leading to inappropriate management [1].
Breast cancer, colon cancer, gastric cancer, and lymphoma are the most frequent secondary neoplasms of the ovary [2, 5–7].
The average age of patients with ovarian involvement for each of the most common forms of cancer that spread to the ovary (intestinal, gastric, and mammary) is significantly lower than that of patients without ovarian spread, presumably because of the greater vascularity in younger women [8]. The non-ovarian primary neoplasm is often diagnosed before an ovarian mass is found [3, 6, 9–11]. Occasionally, a primary tumor may not be discovered until several years after removal of the metastatic ovarian tumor [8].
Metastases can be predominantly solid, solid-containing small cysts, or more rarely, multicystic. The solid component of the mass contains tumoral vessels. Although their pattern can resemble a primary ovarian, knowledge of the main etiologies, most commonly of a previous history of an extra-ovarian cancer, of the routes of dissemination, and of particular radiologic findings must draw the attention to the possibility of metastasis.
The main findings of ovarian metastases are summarized in Table 12.1.
Table 12.1
Main findings of ovarian metastases
1. Etiologies |
1. Breast |
2. Female genital tract |
3. GI tract |
Carcinoma of the stomach, most often Krukenberg tumor |
Intestinal carcinoma |
Tumors of the appendix |
Carcinoid tumors |
Pancreas, bile ducts, liver |
4. Renal, urinary tract (ureter, bladder, urethra) and adrenal tumors |
5. Melanoma |
6. Pulmonary and mediastinal tumors |
7. Extragenital sarcomas |
8. Ovarian involvement by peritoneal tumors |
2. Most common previous history of one of these tumors |
More rarely discovering of ovarian and a malignant extra-ovarian tumor at the same time |
3. Routes of dissemination |
Direct spread (uterus tube mesothelium) |
Through the lumen of the fallopian tube and onto the surface of the ovary |
From more distant sites via blood vessels and lymphatics |
4. Bilateral topography in 2/3 to 3/4 of cases [12] |
While serous carcinomas and undifferentiated carcinomas are also bilateral in 2/3 of cases, primary mucinous and endometrioids are bilateral in 7% and in 13% of cases, respectively [13] |
5. Morphologic characters |
Peripheral capsule with a regular lateral border |
Multiple nodules |
Location of the nodules on the surface of the ovary |
Presence of cysts which often are large and thin-walled despite the absence of cysts in the primary neoplasm |
Follicle-like spaces: |
Gastric, intestinal, appendix |
Small cell |
Malignant melanomas |
6. Vascular findings |
Usual findings of malignancy |
A peripheral vascularization at the arterial phase with a significant contrast uptake at the venous phase (related to a capsule) when present is very suggestive |
7. Very suggestive patterns |
Krukenberg tumor |
Cystic form of metastasis from intestinal carcinoma |
Melanoma (melanocytic type) |
12.2 Metastatic Tumors
12.2.1 Breast
Usually, breast metastases are discovered in the follow up of breast cancer with a median interval of 11.5 months and are related to the stage of cancer [11].
Roughly 75% of ovarian metastases of breast cancer are of the ductal type.
Lobular carcinomas spread to the ovary more often than ductal carcinomas. However, because of the higher frequency of ductal carcinomas, metastases from ductal carcinomas are more common.
Rarely, ovarian metastases of breast carcinoma is a typical Krukenberg; in a series [7] about 1.8% of Krukenberg tumors were of breast origin.
Radiologic Findings are summarized in Table 12.2.
Table 12.2
Imaging findings of ovarian metastases from breast carcinoma
Common findings |
Bilateral two-thirds of cases |
Size <5 cm (85% of cases) [11] |
Solid mass made up of nodules of different sizes [12] |
Containing cysts (20%) |
Surface: |
1. Nodular surface |
2. Smooth-surfaced or bosselated (when the ovary is replaced by tumor) |
Usually accompanied by other intra-abdominal metastases [11] |
Uncommon |
Entirely cystic |
Papillae on gross examination |
Imaging findings are illustrated on the following figures (Figs. 12.1, 12.2, and 12.3).
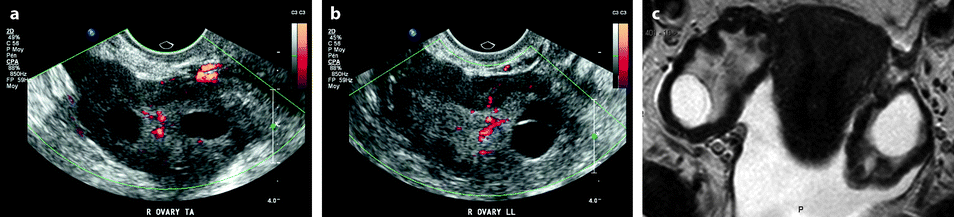
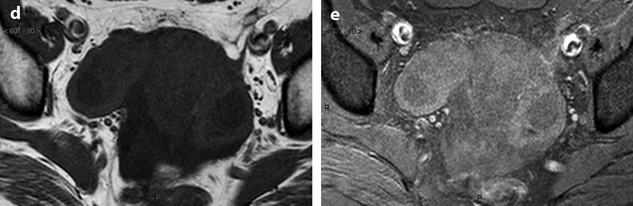
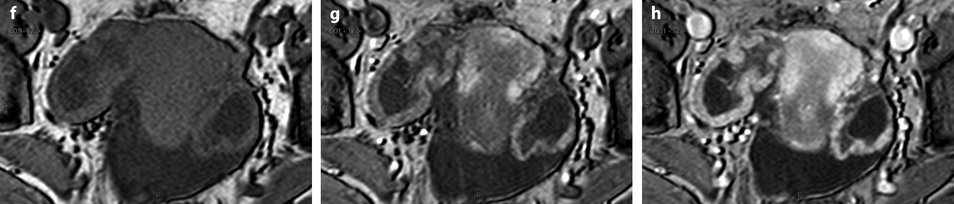
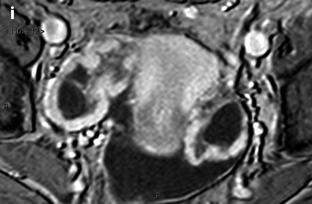
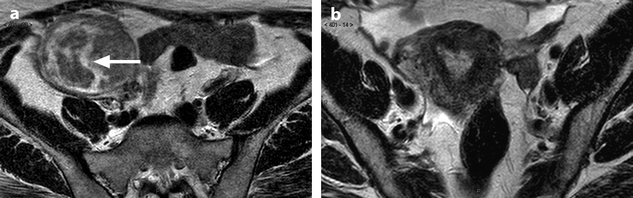
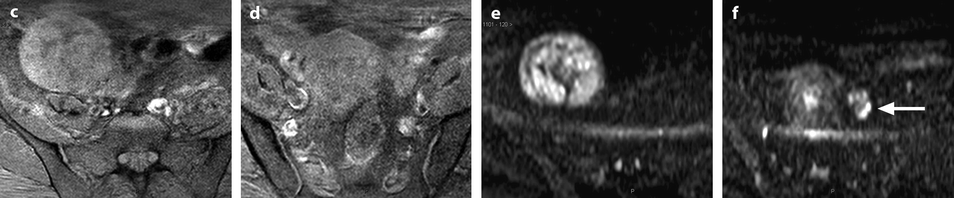
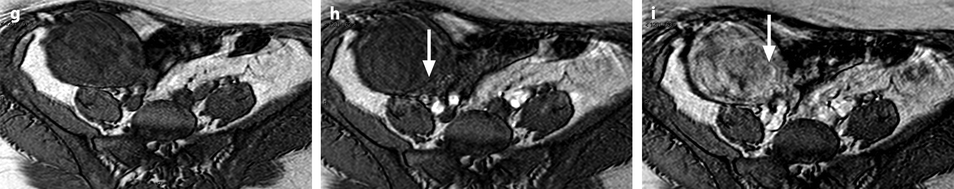
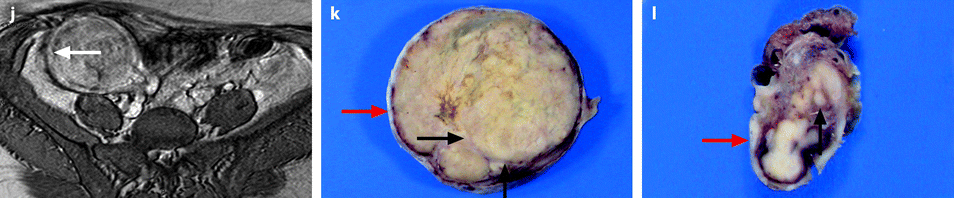
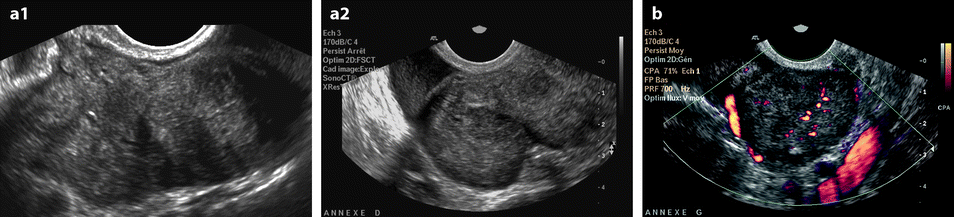
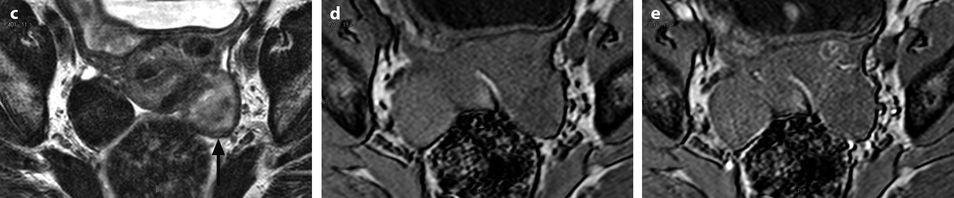
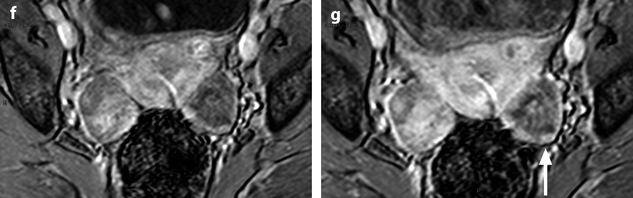




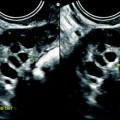
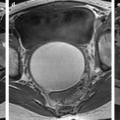
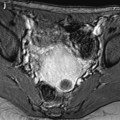
Fig. 12.1
Ovarian metastases of a lobular breast carcinoma T2 N1. Patient under tamoxifen therapy for breast carcinoma treated 3 years ago. EVS with color Doppler of the right ovary (a) and of the left ovary (b) displays a thickened hypoechoic cortex and small cystic structures. MR axial T2 (c) displays a bilateral ovarian mass with a lobulated contour and regular thickening of the cortex that appears with low signal intensity. On T1 (d) and T1 fat suppression (e), signal intensity is more marked in the cortex especially after fat suppression. DMR without injection (f), at the arterial phase (g), at the venous phase (h) and on the delayed (i) show more marked contrast uptake in the cortex. Surgery: Cerebriform ovaries; bilateral adnexectomy was performed. Pathology: (1) Thickening of the ovarian cortex with important fibrosis containing numerous tumoral cells. (2) In the center of the ovaries, the medulla is loose with some tumoral cells




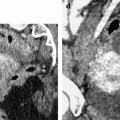
Fig. 12.2
Bilateral ovarian metastasis from breast carcinoma. A 58-year-old woman, 3-cm periareolar lobular invasive carcinoma of the left breast with extension to six axillary lymph nodes operated 4 years ago. Left mastectomy with axillary lymph node dissection, radiotherapy chemotherapy, Arimidex. Left pelvic pain and metrorrhagia for 3 months. CA15-3, CA125 normal. MR axial T2 of the right ovary (a) displays an ovarian mass with a thick peripheral rim corresponding to a capsule of intermediate signal with a well delineated regular lateral border and an irregular medial border, containing some edematous changes (arrow), and on the left ovary (b) a signal close to pelvic muscle. On axial fat suppression of the right ovary (c) and of the left ovary (d), the masses have an overall signal close to pelvic muscles. Diffusion b1000 of the right ovary (e) depicts an overall high signal related to a low ADC. In the left ovary (f) nodules of high signal are displayed on DWI (arrow), which were not visualized in the previous sequences, which highlights the importance of this sequence in the detection of these nodules. DMR of the right ovary without injection (g) at the arterial phase (h) displays arterial vessels at the periphery (arrow). At the venous phase (i), arterial vessels and contrast uptake draw the periphery of nodules like one of these (arrow) very suggestive of metastasis. On the delayed (j), a regular rim of contrast is visualized at the periphery of the ovary (arrow), which is not usually seen in an ovarian carcinoma. CT performed did not demonstrate any extra-ovarian extension. Surgery: bilateral adnexectomy with hysterectomy was performed. Absence of abdominal extension was confirmed. Macroscopic specimen of the right ovary (k) depicts (1) the presence of a thick capsule (red arrow) with a well defined lateral border of variable thickness and (2) separated nodules (black arrows). In the left ovary (l), a well defined capsule is also present (red arrow); white nodules are clearly seen (black arrow). Microscopy: bilateral metastasis of lobular breast carcinoma



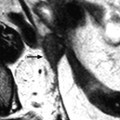
Fig. 12.3
Ovarian metastasis from a breast carcinoma. Association with a subserous leiomyoma; different types of vascularization. A 60-year-old woman, operated 14 years before from a lobular invasive carcinoma of the right breast, treated with tumorectomy, radiotherapy, and chemotherapy. Abdominal pain, CA15-3 increased, ascites. EVS sagittal (a1) through the right ovarian mass displays an upper relatively homogeneous echogenic portion (related to the metastasis) and a lower tissular portion with a strong attenuation related to a subserous leiomyoma. Transverse EVS through the right ovarian mass (a2) and through the left ovarian mass (b) depicts the same type of tumor on both sides. On axial T2 (c), (1) the left ovarian mass has an overall intermediate signal with a very particular thick peripheral rim of low signal intensity (arrow) corresponding to the capsule with an outer regular border and an inner irregular border, (2) the right subserous leiomyoma is of low signal intensity. DMR without injection (d) and at the arterial phase (e) depicts (1) in the left ovarian mass large vessels coming from the left ovarian artery with peripheral regular vessels (2) in the right leiomyoma regular peripheral vessel which are very similar. At the venous phase (f) (1) in the left ovarian mass, a high contrast uptake is visualized in the thickened wall (arrow) which is regular on its lateral border and irregular on its medial border; this finding is very particular to metastases; contrast uptake is much lower in the center. (2) in the right leiomyoma, a peripheral regular rim of contrast uptake associated with a high contrast uptake in the center of the tumor. These differences are even more pronounced on the delayed (g). At pathology: bilateral ovarian metastasis; right serous leiomyoma
12.2.2 Female Genital Tract
12.2.2.1 Endometrial Carcinoma and Endometrial Stromal Sarcomas
Metastases of endometrial carcinoma to the ovary are present in 5–10% of hysterectomies.
Criteria of diagnosis [12]:
1.
Extension of endometrial carcinoma deeply into the myometrium
2.
Tumor present in the fallopian tube
3.
Tumor on the ovarian surface
Metastases of endometrial stromal sarcomas are much more uncommon; metastases from leiomyosarcomas are even more exceptional.
12.2.2.2 Cervical Carcinoma
Ovarian metastases are rare. Ovarian metastasis of adenocarcinoma is a little higher than squamous carcinoma. In a series from the Armed Forces Institute of Pathology, 10% of mucinous adenocarcinomas of the cervix were reported to metastasize to the ovary.
A case of ovarian metastases from invasive verrucous carcinoma (a variant of squamous cell carcinoma) is illustrated (Fig. 12.4).
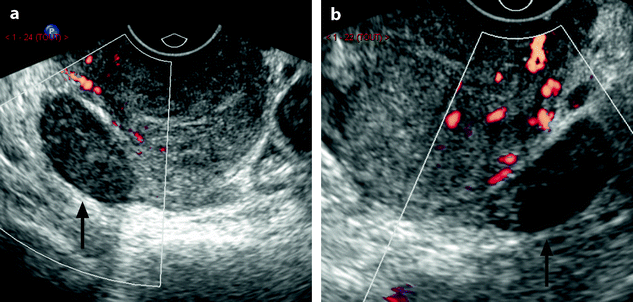
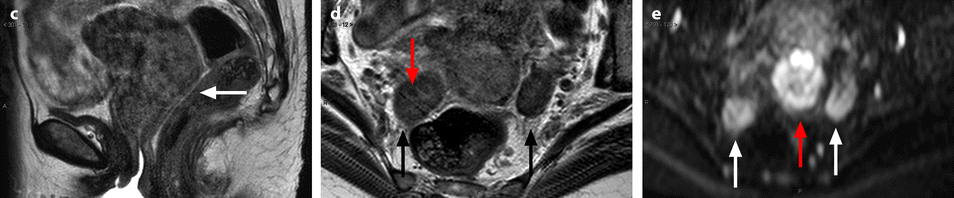
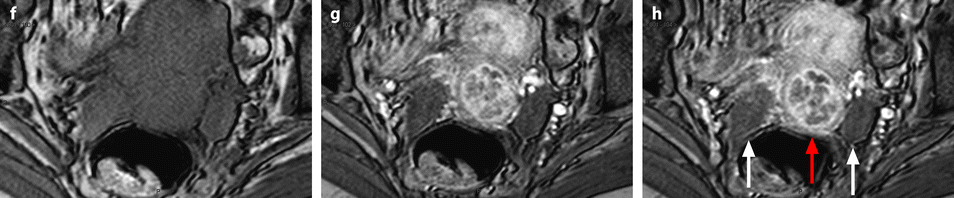
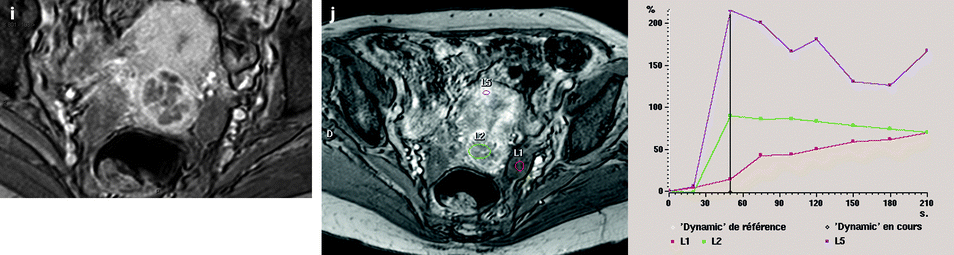
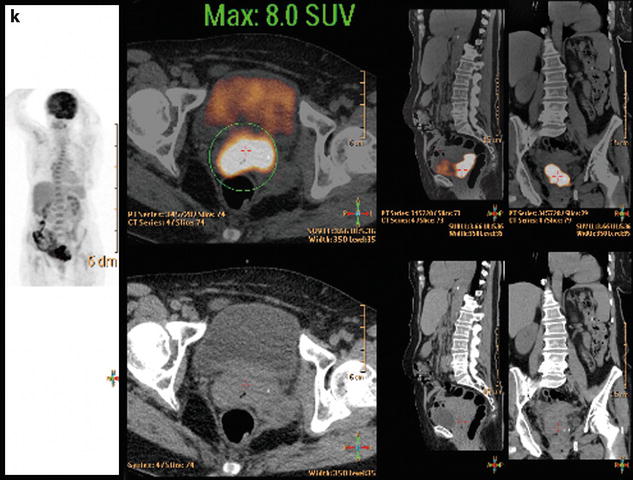





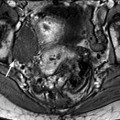
Fig. 12.4
Bilateral ovarian metastasis from carcinoma of the cervix and vagina. A seventy-eight-year-old woman with metrorrhagias. Biopsy of the cervix: well differentiated verucous carcinoma. Transverse EVS with color Doppler (a, b) displays (1) a cervical echogenic mass with color flow related to a cervix carcinoma, (2) a bilateral ovarian mass (arrows) with a homogeneous echostructure, containing some vessels. MR sagittal T2 (c) displays a mass of intermediate signal (arrow) involving the cervix and the vagina typical for a carcinoma. Axial T2 (d) depicts a bilateral enlarged ovary (black arrows) with an overall low signal close to the pelvic muscle, containing in the right ovarian mass small nodules (red arrow). Diffusion using a b1000 sequence (e) shows a high signal intensity in the cervix (red arrow) while intensity in the ovarian masses (white arrows) is much lower. On DMR without injection (f) at the arterial phase (g), tiny vessels are visualized in the ovarian masses while an intense peripheral and central vascularization appears in the cervix; at the venous phase (h) and on the delayed (i), contrast uptake in the ovarian masses is low (white arrows in h), while there is a high contrast uptake in the cervix (red arrow in h) as it is illustrated on the curve (j). PET scan Fig. 12.4 (continued) (k) shows a high signal intensity in the cervix with a maximum SUV of 8 units. Bilateral adnexectomy: ovarian metastasis of cervical carcinoma
12.2.2.3 Tubal Carcinoma
The ovary is involved secondarily in 13% of tubal carcinomas usually by direct extension, sometimes via tubo-ovarian inflammatory adhesions, or by surface implantation on the ipsilateral or contralateral ovary.
Because most tubal carcinomas closely resemble serous, endometrioid or undifferentiated carcinomas of the ovary, microscopic examination often fails to establish whether a carcinoma involving both organs is primary in one or the other.
12.2.2.4 Vaginal Clear Cell Adenocarcinoma
In this case, there is an extensive pelvic spread.
12.2.2.5 Imaging Findings
No particular morphologic features about these different locations has been reported.
12.2.3 GI Tract
12.2.3.1 Carcinoma of the Stomach Including Krukenberg Tumors
Microscopic Definition [14]
Non-ovarian neoplasm in which:
1.
Mucin-filled signet-ring cells account for at least 10% of the neoplasm.
2.
Mucin-free epithelial cells, small glands with flattened lining cells and larger glands with flattened lining cells and larger glands of typical intestinal and mucinous type may be present.
3.
A fibrous stroma ranging from densely cellular to edematous and extracellular mucin may be prominent.
Frequency
Most ovarian metastases of gastric origin are Krukenberg tumors [12].
Two-thirds of Krukenberg arise from the stomach. Carcinomas of appendix, colon, breast, small intestine, and rectum are then the most common primary tumors [14].
The average age of patients is about 45 years.
Macroscopic and radiologic findings are summarized in Table 12.3
.
Table 12.3
Common macroscopic and radiologic findings of Krukenberg tumors
1. Bilateral (80%) usually asymmetrically enlarged [8] |
Involvement of smaller ovary can only be found at microscopic examination [6] |
2. Round or reniform [8] |
4. Typically solid with often ill defined nodules [14] |
Fibrous or edematous gelatinous with focal or diffuse hemorrhage or necrosis |
Containing thin-walled cysts (1/3) filled with mucinous hemorrhage or watery fluid |
Multicystic rarely |
5. Blood vessels occasionally striking in the neoplasm [14] |
6. Ascites (43%) [14] |
Microscopic Findings
Multiple nodules separated by normal stroma are seen in small neoplasm and focally in larger ones.
The tumor is often more cellular at the periphery and edematous or gelatinous centrally. It typically reveals mucin-laden, signet-ring cells strewn individually, in small clusters or in aggregates within a cellular ovarian stroma; occasionally, the stroma has a storiform pattern. Frequent variations include mucin-poor tumor cells, abundant collagen formation, and marked stromal edema. Occasionally, small or large cysts form a conspicuous component of the tumor [8].
Extracellular mucin is often conspicuous and, when associated with scant acellular stroma, gives a distinctive appearance referred to as feathery degeneration [14].
Lutein cells are occasionally present in the stroma, particularly if the patient is pregnant [8].
Radiologic Findings
Radiologic findings in US, CT, and MR are reported in Table 12.4.
Table 12.4
Radiologic findings of Krukenberg tumors
1. US |
Hyperechoic solid pattern (related to intra- and extracellular mucin) |
2. MR and CT |
1. Solid component |
On T2: hypointense fibrous periphery: |
After injection: moderate or strong enhancement different from fibrothecoma or Brenner tumor |
2. Mucinous material |
On T2: hyperintense |
3. Cystic component |
After injection: strong enhancement of the cyst wall |
US Findings (Figs. 12.5 and 12.6)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree