Fig. 1
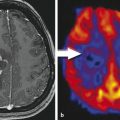
Discriminating glycine from myo-inositol by comparing short and long TE spectra. a shows a spectrum from normal tissue and b from tumor tissue at TE of 30 ms. The signal at 3.56 ppm could be Gly or MI. c shows the normal tissue at a TE of 144 ms, while d shows the repective tumor voxel. Normal tissue is known to contain MI but rather low concentrations of glycine. The lack of the 3.56 signal in c indicates the sufficient suppression of MI at long TE. (Black raw data, red signal estimated by data analyisis, blue residual)
1.2 Measuring 2-hydroxyglutarate
About 70 % of WHO grade II and III gliomas have a mutation of isocitrate dehydrogenase (IDH1 and IDH2). These mutations cause an increased formation of 2-hydroxyglutarate (2-HG) from isocitrate, offering the obvious approach to consider increased 2-HG as a tumor marker visible in vivo by 1H MRS. Increased 2-HG has been observed in vivo in mutated tumors (Andronesi et al. 2012; Choi et al. 2012; Pope et al. 2012), but as for glycine, it cannot be detected easily in standard short TE spectra. Difficulties and potential solutions were recently reviewed (Andronesi et al. 2013) and can be summarized as follows:
Many false positives in standard spectra
Reasonable at optimized TE and adjusted spacing of refocusing pulse followed by dedicated data fitting
Good but clinically not feasible detection using spectral editing either by MEGA-PRESS or 2D methods sensitive to spin–spin coupling
Figure 2 shows data for optimized TE PRESS.
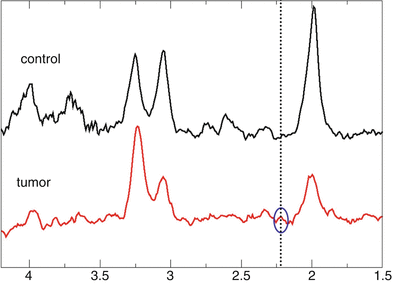

Fig. 2
PRESS spectra obtained at a TE optimized for detection of 2-HG. The dotted vertical line marks the position of the 2-HG signal. The tumor spectrum (lower trace, red) shows a small signal at this position while nothing can be detected in control tissue (upper trace, black)
A recent study hints to a metabolic profile related to the mutation (Esmaeili et al. 2014). This might offer an alternative approach using changes in the phospholipid profile for detection of 2-HG.
2 Other Nuclei
All nuclei with odd mass number do have a spin and a related magnetic moment, i.e., they should be visible with MR spectroscopy. Nuclei other than 1H are also called x-nuclei. Table 1 lists the gyromagnetic ratio γ for nuclei with potential interest in tumor diagnosis. It determines the Larmor frequency ω o for each nucleus in the magnetic field B0 according to ω o = γBo. Keeping in mind that the sensitivity of MR decreases with decreasing Larmor frequency, the bottom row in Table 1 compares the frequency of the x-nuclei to 1H indicating their inferior sensitivity in MR.
Table 1
Values of the gyromagnetic ratios for nuclei of biochemical interest and their ratios to the proton gyromagnetic ratio. Smaller numbers indicate decreased sensitivity in MR
Isotope | 1H | 31P | 13C | 23NA | 15N |
|---|---|---|---|---|---|
γ | 26.7 | 10.8 | 6.73 | 7.08 | −2.7 |
γ/ γH | 1 | 0.40 | 0.25 | 0.27 | 0.10 |
Phosphorous (31P) is of special interest since with almost half the Larmor frequency of 1H it still has a reasonable sensitivity. Actually, 31P MRS was the first method to study tissue metabolism in vivo with MR spectroscopy (see Chap. MR Spectroscopic Imaging).
Further, there is a carbon isotope (13C), which has a natural abundance of 1 % and can also be monitored with MRS. The carbohydrate metabolism can be followed using substances which are enriched (labeled) at specific positions with 13C like [1-13C]glucose, monitoring the fate of the labeled atoms during metabolism. Unfortunately, due to the rather low frequency, the sensitivity of 13C MRS is low. However, there is a method for increasing the amount of MR-sensitive nuclei by hyperpolarization (Ardenkjaer-Larsen et al. 2003), which artificially increases the magnetization for the nuclei of interest by several orders of magnitude compared to the thermodynamic equilibrium. Using this method, imaging of 13C-labeled pyruvate was achieved in several experimental tumor models.
Although not a metabolite, the sodium (23Na) can be monitored with MR as well. The rather high Na concentration in part compensates the low sensitivity. Just for completeness, we added the nitrogen isotope 15N to the table since it has been used for measuring the pathological metabolism by analyzing body fluids in high-resolution NMR spectroscopy.
We will focus on 31P MRS as an easy-to-monitor nucleus with intriguing capabilities to provide information on lipid metabolism which is complementary to 1H MRS. 31P MRS can also be used to measure the intracellular pH and energy metabolism in tissue. In a second part, we will focus on 13C since this nucleus offers a unique field of applications that may become realistic with a broad availability of hyperpolarization. In addition, conventional MRS using 13C-enriched compounds will profit from the potential sensitivity increase due to higher magnetic fields of 7 T and beyond.
2.1 31P MRS
Chapter MR Spectroscopic Imaging already outlined an introduction into the basic principle of 31P MRS and elaborated on the physiological and biochemical information on tumor metabolism. As has already been outlined in this Chapter, its main advantage to 1H MRS is the ability to discriminate between glycerophosphocholine (GPC) and phosphocholine (PCho), adding detailed information to the so-called total choline signal (tCh) obtained from 1H MRS. Also, the signal of the respective ethanolamine compounds can be detected. In addition, 31P MRS measures signals from adenosine triphosphate (ATP) and phosphocreatine (PCr), two important metabolites in energy metabolism. Finally, the position of the signal of inorganic phosphate (Pi), which occurs between 4.6 and 5.5 ppm, is sensitive to pH. With the assumptions that tumor tissue represents densely packed cells, the position of the Pi signal offers an intracellular pH marker. Tissue pH has been discussed as sensitive indicator for treatment effects. These advantages are confronted with the lower sensitivity compared to 1H. Roughly, a factor of 1/2 results from the lower gyromagnetic ratio. Furthermore, an additional factor of 1/3 occurs for creatine and 1/9 for choline compounds, since the main singlet signals in 1H MRS originate from three or nine protons per molecule. Measurements at ultrahigh B0 fields (≥7 T) may compensate for the lower gyromagnetic ratio. Thus, except for the main metabolites, the sensitivity becomes comparable to the sensitivity of 1H MRS. On the other hand, there is the big advantage that no unwanted water and fat signals have to be suppressed during measurement or in data processing. This leads to a well-defined baseline, which considerably improves the accuracy of spectral analysis routines. Since we already stressed the pathophysiological significance of 31P MRS detectable parameters in Chap. MR Spectroscopic Imaging, we will rather concentrate on methodological challenges and potential solutions in the following paragraphs.
2.1.1 Spatial Resolution
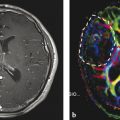
Considering the lower gyromagnetic ratio and the lower number of nuclei in a distinct chemical bonding for metabolites (see above), the inherent sensitivity for a compound like PCr, which is visible in 1H and 31P MRS, should be about 20 % of its 1H sensitivity. This simply requires a 31P voxel volume of five times the 1H-voxel volume or, assuming isotropic resolution, an increase in matrix grid size by almost 75 %. When 1H MRSI and 31P MRSI are obtained with the intention to combine data, a reasonable geometry would match the slice thickness and fit four 1H voxels into one 31P voxel. On top of the larger voxel size, an increased spreading of signal into adjacent voxels caused by the point spread function, which describes the blurring of a point due to coarse k-space sampling, has also been considered. Figure 3 demonstrates the effect: It shows a T2-weighted anatomical slice with a tumor in panel a. Panel b depicts the same slice; however, by digital filtering a blurring was implied which mimics a resolution typical to a 1H MRSI data set obtained with a 16 × 16 matrix at 240 mm2 FOV. Panel c shows this slice at a resolution corresponding to an 8 × 8 × 8 matrix for data acquisition followed by digital resolution enhancement. Obviously, the T2 enhancement marking the tumor is still clearly marked at the 1H MRSI resolution but tends to be blurred across the entire brain at the rather poor k-space sampling employed for 31P MRSI.
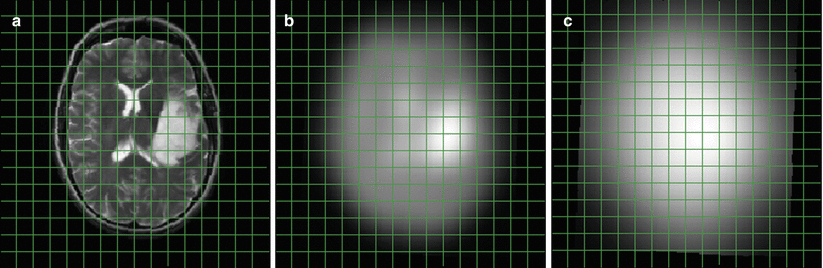

Fig. 3
Blurring of signal intensity due to poor digital sampling. a shows the anatomical image with the tumor in the left hemisphere. b represents the same slice, however, digitally filtered as if the data were obtained with 16 × 16 k-space sampling followed by extrapolation to the image resolution presented in a. c is similar to b, however, with an 8 × 8 × 8 k-space sampling, which is typical in many 31P MRSI studies
2.1.2 Measuring pH
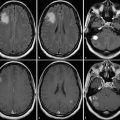
The unique feature of 31P MRS is the ability of the method to detect the pH values in tissue. The effect is based on the pH-dependent equilibrium between HPO4 2− and H2PO4 − with the signal position of inorganic phosphate providing a weighted average of both ions (Prichard et al. 1983). Many studies observed a shift of the Pi signal toward a more alkaline environment (Negendank 1992). With the assumption that this signal originates mainly from intracellular phosphate (Stubbs et al. 1992), the 31P MRS data indicate a reversed pH gradient in tumor tissue (more acidic extracellular) with respective consequences for tumor metabolism and drug efficiency (Stubbs et al. 1994). Figure 4 shows typical 31P MRS spectra from normal-appearing tissue (black) and the tumor (red). The insert at the upper right corner represents the extended Pi region. For the tumor spectra, the Pi signal exhibits a shoulder to the left (lower frequencies or higher pH) and a shift in this direction.
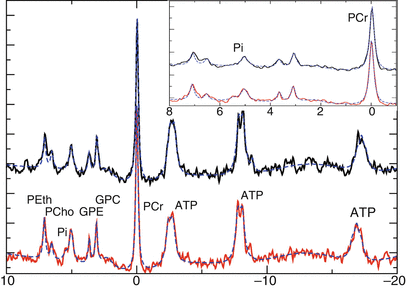

Fig. 4
31P spectra of control (upper trace) and tumor (lower trace) tissue. The insert shows the extended region downfield of PCr, with the position of the Pi signal indicating intracellular pH. x-axes show chemical shift in ppm; y-axes show laboratory units
We want to stress here that partial volume effects prevent from a thorough analysis of tumor pH, leaving the open question whether the lower pH fractions are due to healthy tissue adjacent to the tumor or result from extracellular Pi. A recent publication at least identifies the averaged Pi position as a sensitive marker for treatment (Hattingen et al. 2011).
2.1.3 Measuring Lipid Metabolism
While the tCho signal in 1H MRS just reflects an overall concentration of choline compounds, its main components GPC and PCho can be discriminated with 31P MRS. However, for MRSI data the quantitative analysis in terms of tissue concentrations is severely hampered by partial volume effects as described above. The poor point spread function related to the standard 8 × 8 matrix for data acquisition is causing a sensitivity profile with enhanced signal intensity in the center of the brain. Correction by dividing by tissue content obtained from anatomical MRI after appropriate filtering (see Fig. 3) may account for this, but experimental data on recurrent brain tumors show that the ratio of PCho/GPC, which provides an inherent correction for this, gives better results for comparing tumor to healthy tissue and monitoring treatment (Hattingen et al. 2011; Hattingen et al. 2013). A further advantage of 31P MRS is the ability to observe the ethanolamine analogues to the choline membrane metabolism adding more variables for determining tumor-specific biochemistry.
2.1.4 Energy Metabolism
Metabolite signals from Pi, ATP, and PCr can easily be detected in the 31P MRSI data allowing an estimation of changes in energy metabolism by comparing the ratio of the high-energy phosphates (ATP, PCr) to Pi. Most publications report a consistent drop in this ratio (Negendank 1992; Hattingen et al. 2011; Hubesch et al. 1990; Rutter et al. 1995; Maintz et al. 2002) indicating limited energy supply in tumor tissue.
2.2 13C MRS
2.2.1 Conventional 13C MRS
De Graaf et al. reviewed the unique role of 13C MRS in measuring brain metabolism in vivo (de Graaf et al. 2003) while Ross et al. described preliminary clinical experience in the same journal (Ross et al. 2003). However, due to the poor sensitivity, the in vivo application of 13C MRS to focal lesions in the human brain is even more hampered by limited spatial resolution and partial volume effect than 31P MRS. At least there is one case study (Wijnen et al. 2010) reporting on a malignant glioma. In this study, 13C labeling of lactate and glutamate was observed during an intravenous infusion of 100 % enriched [1-13C]-glucose solution (20 %, w/w). The authors compared 54 × 32 × 29 mm3 voxels, one placed onto the tumor and the other one in the contralateral hemisphere, monitoring the increase in labeled lactate and glutamate. As expected, only glutamate labeling was observed in the normal-appearing tissue while the tumor voxel showed increased lactate labeling and diminished labeling of glutamate. Based on the glutamate labeling in the reference voxel and correcting for the partial volume effect in the tumor voxel, the authors concluded that no glutamate labeling occurs in tumor tissue, indicating no oxidative phosphorylation in tumor cells in accordance with the Warburg effect (Warburg 1956). A more recent study performed in situ 13C labeling with uniformly labeled 13C glucose during surgery (Maher et al. 2012). Perchloric acid extracts from excised tumor specimens were then analyzed by high-resolution NMR spectroscopy. This study enrolled 11 patients with different types of tumors (including metastasis). Despite the variability in tumor type, the authors observed some common features in carbohydrate metabolism: (1) glucose is entering the TCA cycle indicating mitochondrial oxidation of glucose; (2) formation of glycine, glutamate, and glutamine from glucose; (3) in tumor tissue, the TCA cycle oxidizes alternative carbon sources indicating anaplerosis probably due to a high demand for other cell metabolites. In summary, these data highlight the potential of 13C MRS to provide tumor-specific biochemical information, but a noninvasive in vivo protocol for monitoring focal lesions is not available yet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree