This Chapter focuses on the use of MRI to evaluate miscellaneous disorders of the wrist not yet covered in this book. These include tendinopathies, fractures and cartilage lesions, impaction, abutment and impingement syndromes, arthropathies, infection, and soft tissue and bone tumors.
Tendons
The term “tendinitis” is inaccurate because, except in rare cases, inflammatory changes are absent histologically. Rather, most tendons have an accumulation of microscopic injuries that do not heal properly, a form of degeneration that is better termed “tendinosis.” Current theories suggest that pain associated with tendinosis probably results not from inflammation, but from other irritating biochemical substances associated with the injury (1). The hallmark of tendinosis under light microscopy is disruption of the collagen, an increase in ground substance, prominent and numerous tenocytes, and neovascularization (2).
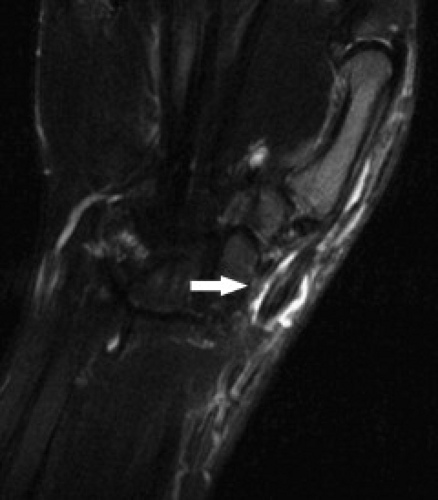
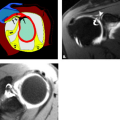
The term tendinopathy has been suggested to refer to general pathology of the tendons, whereas tendinosis suggests poorly healing degeneration. Tenosynovitis refers to inflammation of the tendon sheath and is indicated by tendon sheath fluid that surrounds the tendon completely (Fig. 17.1). The tendon itself may or may not show signs of degeneration or partial tearing. Stenosing tenosynovitis is used to describe the situation when scarring and synovial proliferation leads to local entrapment of the tendon. Finally, peritendinitis refers to abnormality of the tendon and surrounding soft tissues. Additional abnormalities that can affect tendons include partial tears, complete tears, subluxations, and dislocations.
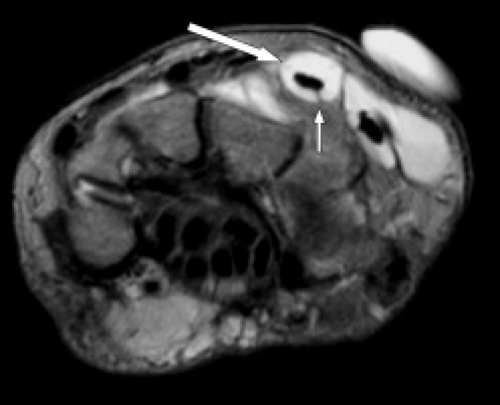 Figure 17.1 Tenosynovitis. Axial T2-weighted image shows fluid completely surrounding the extensor tendons (arrows). |
With the exception of lacerations, most tendon tears occur in degenerated tendons. Tendon degeneration occurring at the wrist shows similar findings as tendons elsewhere in the body: abnormal size, contour, signal, or a combination of any of these (Fig. 17.2) (3).
A common pitfall occurs with any tendon oriented at 55 degrees to the main magnetic field. This may lead to the magic angle artifact on low TE images (T1 images). Evaluation with a higher TE sequence (more T2-weighted) will reveal the signal to be artifactual (Fig. 17.3 A–B).
Tendon Anatomy
Tendon pathology distal to the wrist is described in the Chapter on MRI of finger tendons. Diagrams of the compartments are also included in that Chapter.
The tendons of the wrist are divided into flexor (volar) and extensor (dorsal) groupings. The extensor (dorsal) group can further be divided into six compartments along the dorsal aspect of the wrist (Fig. 17.4; Table 17.1). Overlying these compartments is the extensor retinaculum. Each compartment (regardless of whether it contains one or two tendons) contains a single tendon sheath (4).
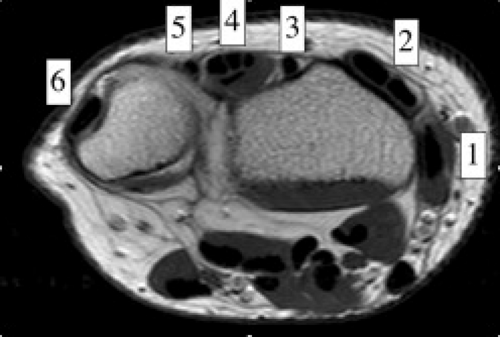 Figure 17.4 Extensor compartmental anatomy. Axial T1-weighted image through the level of the radial carpal joint shows the six compartments of the extensor tendon group (1 through 6). |
Table 17.1 Extensor tendons by compartment (Fig. 17.4) | |
|---|---|
|
Extensor Tendon Pathology by Compartment
Although pathology can affect any of the compartments, the more common conditions are listed subsequently by compartment.
Compartment 1
Tendinosis and tenosynovitis of the extensor pollicis brevis and the abductor pollicis longus tendons at the level of the radial styloid is known as de Quervain’s disease (5) (Fig. 17.5). This entity is seen with higher frequency in individuals with an anatomic variation consisting of a division of the two tendons by a fibrous septum, seen in 20% to 30% of these individuals. Other predisposing factors include repetitive stress from a sport, occupation, or hobby; gout; rheumatoid arthritis; and myxedema (4).
Although usually diagnosed clinically, MRI findings include increased tendon thickness, tenosynovial effusion, and peritendinous edema just proximal to the radial styloid. Increased signal intensity within the tendon is considered a less reliable sign (5). With chronic de Quervain’s disease, there may be intermediate-signal–intensity fibrosing tenosynovitis surrounding the tendons on all imaging sequences. This can lead to decreased range of motion and a trigger finger.
The clinical differential diagnosis includes infection, first carpometacarpal or, scaphotrapezial degenerative arthropathy, flexor carpi radialis tenosynovitis, intersection syndrome (see subsequently), and radial neuritis (4).
Treatment of de Quervain’s tenosynovitis generally consists of conservative measures, including anti-inflammatory medication, rest, avoidance of inciting stresses, splinting, and/or steroid injection. If conservative treatment offers no relief, surgical release of the first dorsal compartment is sometimes performed. Perineural fibrosis around the superficial radial nerve can result from surgical intervention.
Compartment 2
Intersection syndrome is an overuse disorder of the second compartment consisting of peritendinitis occurring 4 to 8 cm proximal to Lister’s tubercle. This corresponds to the crossing point of the first over the second compartment tendons. Physical examination generally reveals pain, tenderness to palpation, redness, and crepitus with flexion and extension.
MRI findings consist of those of peritendinitis: fluid surrounding the tendons and within the tendon sheaths of the first and second compartment starting at the point of crossover and extending proximally. The main differential is de Quervain’s tenosynovitis (4).
This entity can be associated with sporting activities, including rowing, canoeing, racket sports, horseback riding, and skiing (4). Occupational causes of repetitive flexion and extension also can be a cause (6). It generally responds favorably to conservative treatment (7).
It is important to note that because of the location of the tendon crossover, the standard wrist protocol will probably not include the area of interest. For this reason, it is best to include the distal forearm in the field of view. It may also be beneficial to perform the examination using thin oblique sagittal sections (at roughly 60 degrees) paralleling the slope of the lateral aspect of the radius (6).
Compartment 3
The extensor pollicis longus (EPL) tendon is the sole tendon of this compartment. This is the extensor tendon that is most prone to magic angle phenomenon. The EPL tendon can be affected by chronic friction from the adjacent Lister’s tubercle leading to tenosynovitis and rupture. Another cause of third-compartment dysfunction is a distal radial fracture. A delayed rupture of the tendon following a fracture is thought to be the result of one of two mechanisms: mechanical irritation of the tendon caused by a sharp edge of the fractured bone or a direct microvascular compromise of the poorly vascularized tendon (8).
Compartments 4 and 5
Tenosynovitis is common in the extensor digitorum compartment. Ruptures of the extensor digitorum, extensor digiti indicis, and extensor digiti minimi are
usually divided into closed and open injuries (lacerations). In these wrist extensor compartments, ruptures are usually related to lacerations that can be associated with proximal tendon retraction (4).
usually divided into closed and open injuries (lacerations). In these wrist extensor compartments, ruptures are usually related to lacerations that can be associated with proximal tendon retraction (4).
Compartment 6
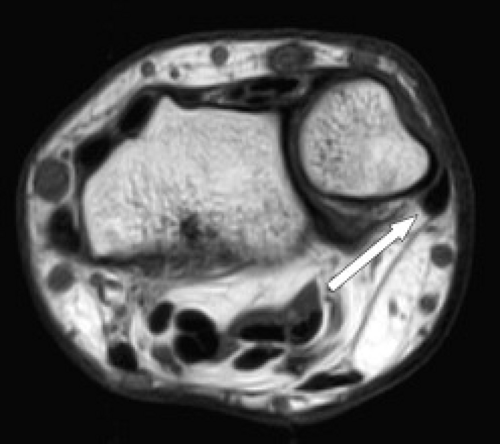
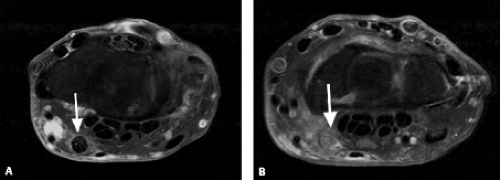
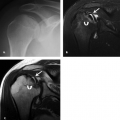
Tendinopathy and partial tears of the extensor carpi ulnaris tendon are fairly common (Fig. 17.6). This may be related to acute trauma, repetitive motion, or an ununited ulnar styloid fragment. Athletes may also be affected, particularly those who play baseball, golf, or racquet sports. Subluxations or dislocations can occur, especially when the subsheath is torn, and occur with the wrist in supination, ulnar deviation, and palmar flexion. The overlying extensor retinaculum may be thickened or disrupted. Subluxations and dislocations also occur more often in rheumatoid arthritis because of distal radioulnar instability (Fig. 17.7). Ruptures rarely occur in athletes (9). Instability or chronic stenosing tenosynovitis can lead to partial tears (4). Tendinitis is
usually treated conservatively with medication, splinting, and injections of steroid and anesthetic. Rupture of the tendon or retinaculum is treated with surgical reconstruction.
usually treated conservatively with medication, splinting, and injections of steroid and anesthetic. Rupture of the tendon or retinaculum is treated with surgical reconstruction.
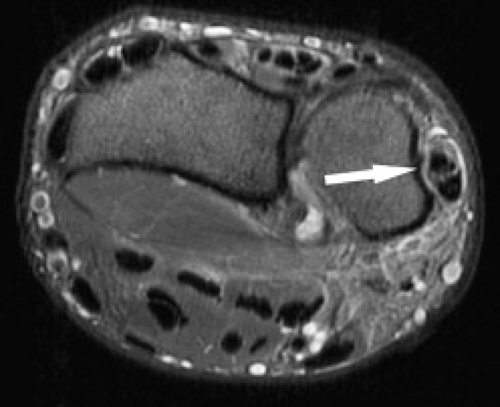 Figure 17.6 Tendinopathy and tear. Axial fat-suppressed T2-weighted image shows a longitudinal tear and tendinopathy of the extensor carpi ulnaris tendon (arrow). |
Flexor Tendon Anatomy
Some flexor tendon pathology has already been reviewed in the previous Chapter on the carpal tunnel. For completeness, some key points regarding the flexor tendons are presented here. The deep and superficial flexor tendons and the flexor pollicis longus tendons traverse the volar side of the wrist through the carpal tunnel. These can be divided into those that traverse the wrist within the carpal tunnel and those that do not. Within the carpal tunnel travel the flexor digitorum superficialis, flexor digitorum profundus, and the flexor pollicis longus tendons—the main flexors of the fingers. Outside the carpal tunnel travel the main flexors of the wrist, including the flexor carpi radialis, the flexor carpi ulnaris, and the palmaris longus tendons. The palmaris longus tendon presents the most anatomic variation of the wrist tendons (4).
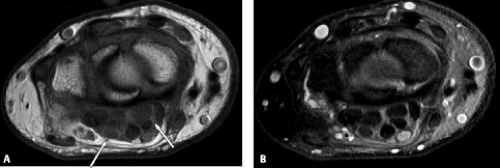
Complete ruptures of the digital flexor tendons are more often the result of laceration because closed ruptures are rare (10). When closed ruptures do occur, it is usually in the setting of an underlying disorder such as rheumatoid arthritis (Fig. 17.8 A–B), osteoarthritis, scaphoid nonunion, Kienböck’s disease, hook-of-the-hamate fractures, and carpal dislocations. Occasionally athletes will sustain a closed rupture from a hyperextension injury (4).
Osseous and Cartilaginous Injury
Fractures of the distal forearm and carpal bones are common after a fall on an outstretched hand. Although most fractures are evaluated with conventional radiography, MRI can play an important role because of its superior sensitivity. MRI should be considered when there is a concern for concomitant ligament injuries or when a fracture is suspected clinically but not shown on radiographs (11). MRI can also be useful to follow complications after fracture, including malunion, nonunion, and cartilage damage.
Distal Radial Fractures
The most common distal radial fractures include: (i) Colles fracture characterized by a dorsal tilt of the radius and typically occurs 2 to 3 cm from the articular surface in the distal radial metaphysis often extending to the articular surface; (ii) Barton’s fracture also has a dorsal tilt and occurs on the dorsal or volar (reverse Barton’s) lip of the radius with associated subluxation of the carpus; (iii) Smith’s fracture characterized by an extra- or intra-articular fracture with a volar tilt of the radial
articular surface; (iv) Chauffer’s fracture (also called Hutchinson or bumper fracture) occurs in the radial styloid; (v) die-punch fracture refers to fractures that involve the lunate fossa of the distal radius; and (vi) Galeazzi fracture–dislocation consists of a fracture of the distal third of the radius that may extend to the articular surface, is often dorsally displaced, and is associated with dorsal ulnar dislocation of the distal radioulnar joint.
articular surface; (iv) Chauffer’s fracture (also called Hutchinson or bumper fracture) occurs in the radial styloid; (v) die-punch fracture refers to fractures that involve the lunate fossa of the distal radius; and (vi) Galeazzi fracture–dislocation consists of a fracture of the distal third of the radius that may extend to the articular surface, is often dorsally displaced, and is associated with dorsal ulnar dislocation of the distal radioulnar joint.
Surgical treatment of wrist fractures is primarily dependent on fracture location and any displacement or angulation. Other factors include the age and physical demands of the patient and the degree of osteopenia present. Options for treatment include immobilization with casting, internal plate fixation, and percutaneous pin fixation. Those that appear stable with minimal displacement or impaction can be treated conservatively with closed reduction in a cast.
If an articular fracture unites with a surface incongruity of more than 2 mm, surgery is indicated to avoid the risk of arthropathy. Those fractures that require open reduction and internal fixation (ORIF) include Barton’s, reverse Barton’s, and radial styloid fractures. Compression fractures of the radial articular surface or fractures with displaced and rotated fragments also require ORIF. Malunion of the distal radius is more common in the wrist than in any other site in the body, occurring in 5% of patients. If the radius has not been adequately reduced after an impaction fracture, continued radial shortening will result in positive ulnar variance and can lead to ulnocarpal impaction syndrome.
After a radial fracture, the cartilage can be damaged, leading to radiocarpal arthropathy (Fig. 17.9). MRI allows for early diagnosis of the cartilage damage. The triangular fibrocartilage, proximal-row intrinsic carpal ligaments, and extrinsic carpal ligaments may also be torn in the presence of a fracture of the distal forearm. Osseous displacement, malunion, and nonunion can be identified using either MRI or computed tomography. The soft tissue abnormalities are best seen on MRI unless artifact-inducing hardware is present. MR arthrography can be used to evaluate for ligament or triangular fibrocartilage damage in those situations in which the hardware would produce significant artifact.
Carpal Fractures
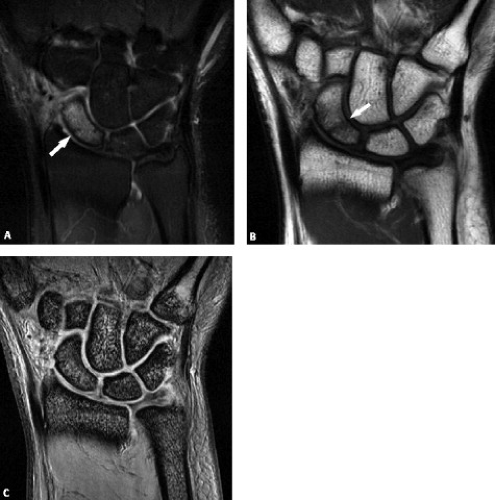
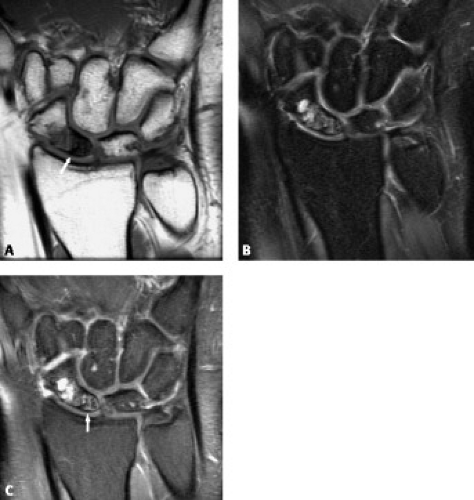
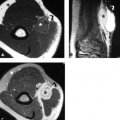
Although carpal fractures are less common than distal radial fractures, they are more likely to require MRI evaluation as a result of their radiographic obscurity. Scaphoid and triquetral fractures are the most common of the carpal fractures (11). When considering carpal fractures, one should rely on both the T1-weighted and fat-suppressed T2-weighted images. Although gradient sequences offer higher resolution, they can be insensitive to bone marrow edema (Fig. 17.10 A–C).
Scaphoid fractures often occur during hyperextension of the wrist like with a fall on the outstretched hand. Most occur at the waist with the remainder mostly involving the proximal pole. Radiographs are well known to be insensitive to acute scaphoid fractures. For a patient with a strong clinical concern for a fracture (based on mechanism or anatomic snuffbox pain) and a negative radiograph, the traditional approach has been to immobilize the wrist and perform repeat radiographs in 7 to 10 days. MRI can avoid unnecessary immobilization and the associated loss of productivity by excluding a fracture at the initial presentation. MRI also allows immediate detection of additional injuries (such as ligament tears).
Scaphoid fractures are usually treated with immobilization if stable. These include incomplete and nondisplaced fractures and those without displacement. Fractures with any displacement or comminution or those associated with carpal dislocation are considered unstable and are generally treated surgically. There are, however, numerous classifications systems used to determine stability and it is therefore best to precisely describe the fracture location, orientation, and angulation as well as describe any displacement that may be present.
Because the arterial supply of the scaphoid usually enters the bone distally, a fracture at the proximal pole or waist puts the proximal fragment at risk for avascular necrosis (osteonecrosis). The risk of osteonecrosis is directly related to the fracture location with a fracture of the middle third having a 30% chance of osteonecrosis, whereas a fracture of the proximal fifth nearly always leads to osteonecrosis (11). Even in the absence of complicating osteonecrosis, the healing time of a more proximal fracture will be prolonged because of its tenuous blood supply.
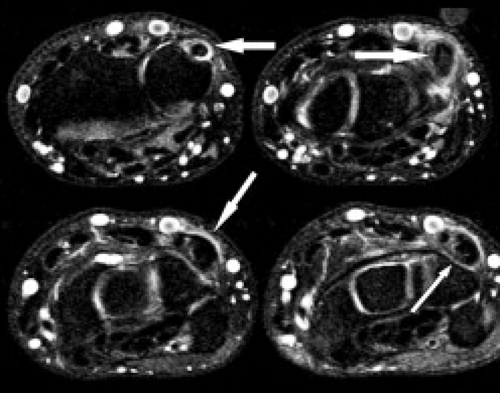
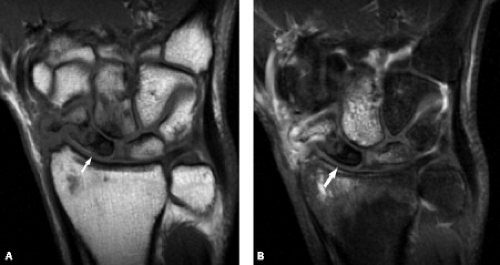
Radiographs are insensitive to the detection of early osteonecrosis, because sclerosis of the proximal pole may not correlate with necrosis. A transient diminished vascularity of the proximal pole can result in less osteopenia compared with the distal pole. The relative increase in density in the proximal pole can falsely suggest osteonecrosis (11). MRI can play a key role in the workup of suspected osteonecrosis after a scaphoid fracture (Fig. 17.11 A–B). Noncontrast MRI can generally confirm the presence of viable bone by showing normal T1 signal intensity. Low signal on both T1- and T2-weighted sequences is seen with osteonecrosis, whereas low T1 and high T2 signal is less clear, possibly the result of edema or osteonecrosis (Fig. 17.12 A–C). Contrast can be very helpful in these confusing situations. When contrast is followed by fat-suppressed T1-weighted sequences, a lack of enhancement of the proximal scaphoid bone marrow indicates lack of blood perfusion (12). On the other hand, contrast uptake verifies perfusion even in the presence of low signal intensity on T1 weighting. Treatment options for osteonecrosis include vascularized pedicle graft surgery.
Aside from osteonecrosis, other problems that can follow a scaphoid fracture include delayed union, nonunion, deformity, carpal instability, carpal tunnel syndrome, reflex sympathetic dystrophy, and osteoarthritis (12). Scaphoid nonunion is the term used when the fracture has not healed after 6 months. Factors contributing to nonunion include severity and type of the fracture, displacement of the fracture of more than 1 mm, and an associated dorsal intercalated segment instability (DISI) and/or ligamentous disruption. Delayed diagnosis and
poor immobilization of a fracture also contribute to nonunion. The nonunion can be shown on MRI by a lack of bridging bone across the fracture, sometimes with T2 hyperintensity at the nonunion site. Osteonecrosis is common in the setting of a nonunion and its presence in the proximal fragment is the primary prognostic factor in predicting successful surgical treatment of scaphoid nonunion.
poor immobilization of a fracture also contribute to nonunion. The nonunion can be shown on MRI by a lack of bridging bone across the fracture, sometimes with T2 hyperintensity at the nonunion site. Osteonecrosis is common in the setting of a nonunion and its presence in the proximal fragment is the primary prognostic factor in predicting successful surgical treatment of scaphoid nonunion.
The association of scaphoid fracture and carpal instability is a complex concept that is worth considering. In the anatomic state, the scaphoid assumes a palmar-flexed attitude. That is, the distal pole of the scaphoid has a tendency to flex in the volar (palmar) direction. The normal axial load exerted by the forearm will accentuate this. The adjacent lunate and triquetrum meanwhile act to counterbalance this force with an opposite dorsal attitude.
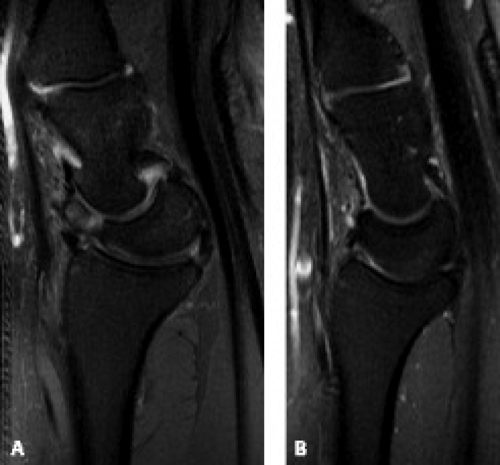
In the setting of an unstable scaphoid fracture, the distal fragment continues to undergo palmar flexion, whereas the proximal fragment experiences dorsal forces through its association with the lunate and triquetrum. With disruption of the surrounding ligaments (such as occurs in perilunate dislocations), the scaphoid will flex while the adjacent lunate extends leading to a DISI pattern of instability revealed on lateral radiographs as an abnormal dorsal tilt of the lunate (Fig. 17.13 A–B) (13). One should keep in mind that even a normal lunate often appears more dorsally tilted on MRI than on radiographs. Additionally, this dorsal tilt is exacerbated when the wrist is in ulnar deviation or in the prone position. Because of these factors, one should be cautious to make the diagnosis of DISI solely on an MR examination without the benefit of radiography (14).
Fractures of the scaphoid waist may collapse with dorsal angulation of the fragment, resulting in a flexion (humpback) deformity that is best seen in the sagittal plane. Osteotomies are performed when a humpback deformity is discovered. Nonunion leads to a pattern of instability and articular degeneration known as scaphoid nonunion advanced collapse (SNAC) wrist. Four progressive stages of SNAC wrist have been identified, including arthritis of the radial styloid, extension of the arthritic change to the scaphoid fossa of the radius, capitolunate arthritis, and diffuse carpal arthritis. Treatment of nonunion includes bone graft, midcarpal fusion, or arthrodesis, depending on the severity of the secondary arthropathy.
MRI can also be helpful in identifying other carpal fractures, particularly radiographically occult fractures as well as bone contusions and their associated soft tissue abnormalities. A fracture line that is not apparent on radiographs can often be seen on MRI as a T2 hyperintensity and a T1 hypointensity. Computed tomography can be used in a complimentary fashion to depict cortical detail, small fragments, and displacement of fractures.
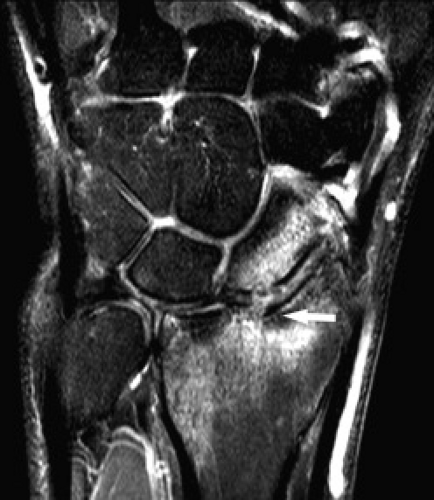
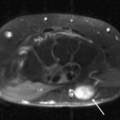
Hamate fractures may involve the body and/or the hook (hamulus) (Figs. 17.14 and 17.15). A hook-of-the-hamate fracture commonly results from direct pressure from a bat, club, racket, or stick on the dominant hand during sports. Fractures at the base of the hook of the hamate tend to heal well, whereas fractures at the palmar aspect of the hook of the hamate require resection.
Capitate fractures, like scaphoid fractures, are at risk of resulting in osteonecrosis after fractures of the proximal pole because the blood supply enters at the waist of the bone.
Capitate fractures, like scaphoid fractures, are at risk of resulting in osteonecrosis after fractures of the proximal pole because the blood supply enters at the waist of the bone.
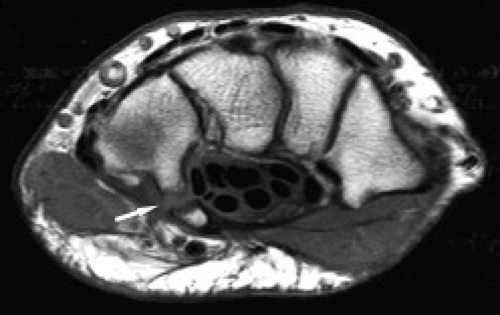
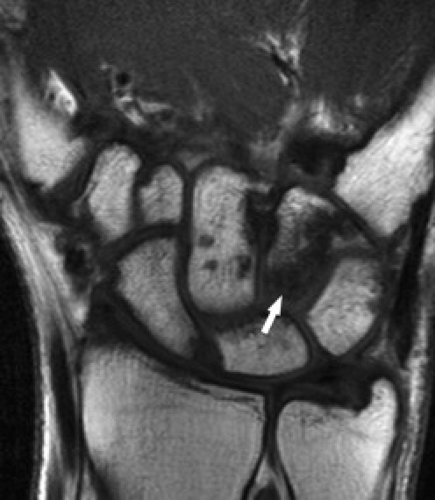 Figure 17.14 Hamate fracture. Coronal T1-weighted image shows an irregular fracture line through the hamate (arrow) that was not detected on radiography. |
Lunate fractures are not common but are also associated with a fall on an outstretched hand. Osteonecrosis of the lunate, also known as Kienböck’s disease, or lunatomalacia, is thought to represent a chronic manifestation of lunate stress from factors such as a short ulna (negative ulnar variance), a squared or oblong lunate morphology, or a vascular insult related to acute or repetitive microtrauma (11). Kienböck’s disease is most commonly seen in males between 20 and 40 years of age.
The most common classification system for staging of Kienböck’s disease is that of Lichtman who described four stages (15) (Table 17.2). Stage I disease consists of a normal radiograph with wrist pain. The MRI is positive for osteonecrosis at this stage and is very useful for early diagnosis. Stage II is manifest as sclerosis of the lunate on radiographs that may be accompanied by collapse of the radial side of the lunate. Stage III Kienböck’s disease demonstrates lunate collapse. Stage IIIA differs from Stage IIIB by the absence of fixed scaphoid rotation and proximal migration of the capitate seen in the latter stage as a result of scapholunate ligament disruption. Stage IV is characterized by all of findings in Stage III with the addition of arthritic changes in the carpus. In all stages, there is usually complete involvement of the marrow with variable signal intensity on fluid-sensitive sequences ranging from hypo- to hyperintensity (Fig. 17.16). The fracture line may also be seen. Beyond Stage I, MRI is not necessary for diagnosis but can be used to show involvement of cartilage and adjacent ligaments or associated carpal tunnel syndrome. Carpal tunnel compression can result from pressure of an expanding lunate causing the flexor retinaculum to collapse on itself. MRI can also be useful to monitor the progression of osteonecrosis. Early identification of lunate osteonecrosis allows for splinting and immobilization that may reverse the necrosis. The later stages of this disease are treated with various procedures. These include lunate unloading procedures such as radial wedge osteotomy to shorten the radius, ulnar lengthening, carpal fusion, and/or proximal-row carpectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree