The elbow is susceptible to a number of disease processes that may or may not be specific to, or commonly encountered in, this articulation. Among these miscellaneous conditions of the elbow, nerve abnormalities, synovial pathology, and neoplasms can prove to be diagnostic challenges to both the clinician and radiologist. This Chapter serves to review the pertinent anatomy relating to these topics as well as MRI characteristics that may prove to be instrumental in avoiding pitfalls in imaging and clinical diagnoses.
MRI of Elbow Nerves
The three primary nerves about the elbow, the ulnar, radial, and median nerves, are susceptible to a variety of compressive neuropathies at the elbow and proximal forearm. The diagnosis is often based on a combination of the clinical history of the patient, physical examination findings, and, in some cases, electrodiagnostic studies. Accurate diagnosis is often confounded by the nonspecific nature of the findings provided by the aforementioned studies and the complex anatomy of the nerves about the elbow resulting in many sites of potential compression that are often multiple in nature. Although MRI is by no means a diagnostic panacea in the setting of nerve disorders about the elbow, it does have a role in nerve assessment that includes the detection of external processes that could cause mass effect and nerve compression, the detection of end-organ manifestation of nerve pathology in the way of muscle denervation, and the exclusion of other pathologic conditions that could mimic a neuropathy at the elbow.
Ulnar Nerve Anatomy
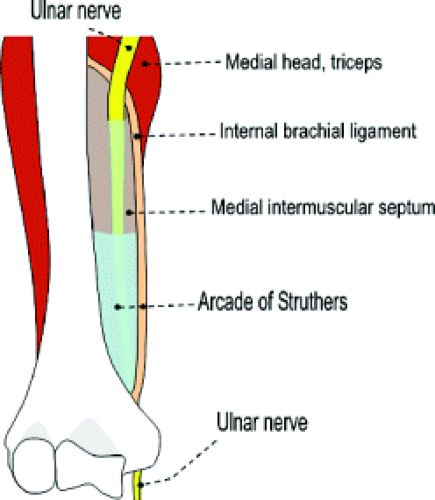
The ulnar nerve is formed by the medial cord of the brachial plexus and arises from the C8 and T1 nerve roots (Fig. 13.1). In some instances, it can receive fibers from the C7 nerve. It follows a distal course medial to the axillary and brachial arteries, interposed between artery and vein, to the level of the midarm. Here it pierces the intermuscular septum and occupies a position anterior to the medial head of the triceps within the posterior compartment of the arm.
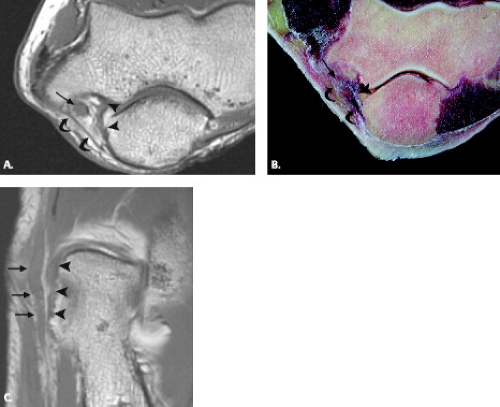
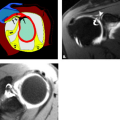
Approximately 8 cm proximal to the medial epicondyle, the nerve may pass under a fibrous tunnel called the Arcade of Struthers, present in 70% of individuals and formed by a band that connects the medial intermuscular septum to the tendon of the medial head of the triceps. At the level of the elbow, the ulnar nerve is housed in the cubital tunnel (Fig. 13.2). The cubital tunnel is a fibro-osseous conduit along the posterior aspect of the medial epicondyle. This fibro-osseous space is formed by the medial epicondyle anteriorly and the medial margin of the trochlea and olecranon laterally. The posterior bundle of the ulnar collateral ligament forms the floor of the cubital tunnel and the arcuate ligament the roof. The arcuate ligament may be absent in up to 23% of subjects (1). Fatty tissue usually surrounds the ulnar nerve and posterior recurrent ulnar artery as they pass through the cubital tunnel.
The ulnar nerve continues distally occupying a position between the humeral and ulnar heads of the flexor carpi ulnaris muscle. Ultimately, the nerve pierces the deep flexor pronator aponeurosis and travels between the flexor carpi ulnaris and the flexor digitorum profundus toward the wrist (2–4). The nerve may be identified in the proximal forearm along the medial aspect of the ulna within a small triangular focus of fat just lateral to the flexor digitorum superficialis.
The ulnar nerve innervates the skin and muscles of the ulnar side of the forearm and hand. This includes the flexor carpi ulnaris and half of the flexor digitorum profundus muscles.
Radial Nerve Anatomy
The radial nerve is a direct continuation of the posterior cord of the brachial plexus comprised of C5 through C8 and T1 nerve roots. It is the largest branch of the brachial plexus and descends between the anterior axillary and brachial arteries and the posterior subscapularis and latissimus dorsi tendons (Fig. 13.3).
The nerve courses distally between the long and medial heads of the triceps, passing obliquely across the back of the humerus and ultimately residing in a shallow groove deep to the lateral head of the triceps muscle. At the lateral side of the humerus, it pierces the lateral intermuscular septum approximately 10 cm proximal to the lateral epicondyle and travels between the brachialis and brachioradialis muscles (5).
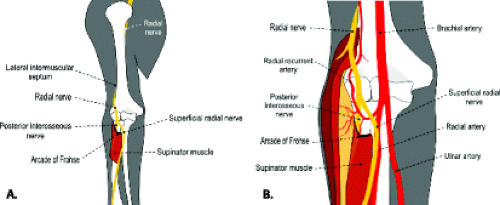
At the proximal margin of the supinator muscle, just superficial to the radiohumeral joint, the nerve divides into the superficial and deep branches. The superficial branch of the radial nerve continues distally deep to the brachioradialis, between it and the supinator muscle. The deep motor branch, commonly referred to as the posterior interosseous nerve, passes between the superficial and deep portions of the supinator muscle (Fig. 13.4).
The posterior interosseous nerve courses distally through a fibromuscular tunnel termed the radial tunnel. The radial tunnel begins at the level of the capitellum near the proximal border of the supinator and
extends to the distal margin of the supinator muscle (6, 7). The supinator tunnel refers to the distal portion of the radial tunnel and corresponds to the fatty space between the superficial and deep portions of the supinator muscle through which the posterior interosseous nerve passes. The roof of the radial tunnel is formed by fibrous bands of adjacent muscles, the Leash of Henry (recurrent radial artery vessels), and the Arcade of Frohse. The Arcade of Frohse is the fibrous arch, present in 35% of individuals, formed by the proximal border of the superficial portion of the supinator and demarcates the proximal portion of the supinator tunnel (8). The radial tunnel is prone to compression at four primary sites: fibrous bands forming the proximal roof of the tunnel, the Leash of Henry, the Arcade of Frohse, and by the extensor carpi radialis brevis (ECRB) muscle (6). Various terms have been used to describe impingement of the posterior interosseous nerve within the radial tunnel, including radial tunnel syndrome, supinator syndrome, posterior interosseous nerve syndrome, and resistant tennis elbow syndrome.
extends to the distal margin of the supinator muscle (6, 7). The supinator tunnel refers to the distal portion of the radial tunnel and corresponds to the fatty space between the superficial and deep portions of the supinator muscle through which the posterior interosseous nerve passes. The roof of the radial tunnel is formed by fibrous bands of adjacent muscles, the Leash of Henry (recurrent radial artery vessels), and the Arcade of Frohse. The Arcade of Frohse is the fibrous arch, present in 35% of individuals, formed by the proximal border of the superficial portion of the supinator and demarcates the proximal portion of the supinator tunnel (8). The radial tunnel is prone to compression at four primary sites: fibrous bands forming the proximal roof of the tunnel, the Leash of Henry, the Arcade of Frohse, and by the extensor carpi radialis brevis (ECRB) muscle (6). Various terms have been used to describe impingement of the posterior interosseous nerve within the radial tunnel, including radial tunnel syndrome, supinator syndrome, posterior interosseous nerve syndrome, and resistant tennis elbow syndrome.
Proximally, the radial nerve innervates the medial and long heads of the triceps and the anconeus muscles. In the distal arm, the nerve supplies motor branches to the brachialis, brachioradialis as well as the extensor carpi radialis longus and brevis muscles. It also supplies the elbow and skin in the radial aspect of the proximal forearm. The posterior interosseous nerve usually provides motor innervation to the supinator muscle proximally and extensors of the hand and wrist distally, although there is significant variability regarding innervation patterns of the extensor muscles by the radial and posterior interosseous nerves (6).
Median Nerve Anatomy
The median nerve arises from the lateral (C5 to C7 nerve roots) and medial (C8 and T1 nerve roots) cords of the brachial plexus. It courses distal in the arm medial to the biceps muscle and anterior to the brachial artery. At the level of the elbow, the median nerve lies deep to the lacertus fibrosis along the medial aspect of the brachialis muscle. The median nerve is slightly medial to the brachial artery and biceps tendon at the level of the elbow. The brachial artery bifurcates at the level of the radial head into radial and ulnar arteries, which continue distally with the superficial branch of the radial nerve and ulnar nerve, respectively. The median nerve enters the forearm between the two heads of the pronator teres muscles and passes superficial to the ulnar head. It is then separated from the ulnar artery and passes deep to the fibrous arch formed by the two heads of the flexor digitorum superficialis. As it courses distally in the forearm, it maintains a position between the flexor digitorum superficialis and flexor digitorum profundus. Approximately 5 cm proximal to the flexor retinaculum, it emerges from behind the lateral edge of the flexor digitorum superificialis assuming a more superficial position (Figs. 13.5 and 13.6) (2, 3, 9).
Muscular branches of the median nerve at the level of the elbow provide motor innervation to the pronator teres and flexor muscles. Visualization of the median nerve can be difficult, particularly if there is little surrounding perineural fat. The nerve can often be seen between the pronator teres and brachialis muscles on axial MR images at the proximal margin of the elbow.
The anterior interosseous nerve is the major branch of the median nerve in the forearm, arising 5 to 8 cm distal from the elbow at the inferior margin of the pronator teres. The nerve courses distally along the interosseous membrane, deep to the flexor digitorum profundus and flexor pollicis longus. It is considered a purely motor nerve and supplies the deep ventral muscles of the forearm (radial half of the flexor digitorum profundus, flexor pollicis longus, and pronator quadratus muscles). Impingement of the anterior interosseous nerve, termed Kiloh Nevin syndrome, may mimic pronator syndrome clinically.
MRI Anatomy of Nerves
MRI can provide a means to visualize nerves as small as the median and ulnar nerves, although smaller nerves can be difficult to distinguish from adjacent vascular structures. On T1-weighted images, the nerve should be intermediate in signal intensity, isointense to muscle. On fluid-sensitive sequences, the nerve may brighten slightly in signal intensity as compared with muscle, but
should not rival the signal of simple fluid (7, 10). Moreover, in many cases, the nerves should be surrounded by a rim of fat. Based on these observations, comparison of T1-weighted and fluid-sensitive sequences can help to differentiate the small nerve from adjacent vessel (11).
should not rival the signal of simple fluid (7, 10). Moreover, in many cases, the nerves should be surrounded by a rim of fat. Based on these observations, comparison of T1-weighted and fluid-sensitive sequences can help to differentiate the small nerve from adjacent vessel (11).
Routine axial images with the elbow in full extension and the wrist supinated allow consistent identification of all major nerves. Specialized axial views with pronation of the forearm may be helpful for better characterization of the median and radial nerves (12). In the axial plane, a normal nerve has a smooth round or ovoid shape.
With regard to the MRI of specific nerves, the ulnar nerve is the most conspicuous nerve at the elbow and is highlighted by fat throughout its course in the elbow and forearm (7, 9). In particular, fat signal should be present throughout the cubital tunnel surrounding the intermediate-signal–intensity ulnar nerve. A few small dot-like structures adjacent to the nerve represent posterior recurrent ulnar vessels, which are hypointense on T1-weighted images and of variable signal intensity on T2-weighted images depending on the degree of blood flow. The arcuate ligament, forming the roof of the tunnel, is seen as a slender hypointense band. Occasionally, specialized axial views with the elbow in flexion may be useful for further characterization of the ulnar nerve and cubital tunnel. Images obtained during elbow flexion can reveal narrowing of the cubital tunnel, attenuation of the perineural fat, bulging of the medial head of the triceps, and flattened morphology of the ulnar nerve, all of which could be seen in the clinical entity of cubital tunnel syndrome (13, 14).
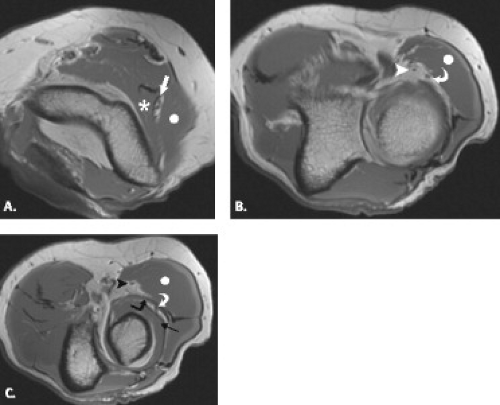
The radial nerve is most consistently seen at the proximal margin of the elbow surrounded by a strip of fat as it courses between the brachialis and brachioradialis muscles (7, 13, 14). Its two branches, the superficial radial nerve and the deep, posterior interosseous nerve, can be identified at the level of the radiocapitellar joint but may be difficult to distinguish from adjacent recurrent vessels. The Arcade of Frohse may be seen at the proximal edge of the superficial head of the supinator muscle and appears as a low-signal–intensity band. The posterior interosseous nerve can be identified more distally when sufficient amounts of fat are present to separate the superficial and deep heads of the supinator muscle.
The median nerve is often poorly visualized at the elbow as it courses between the pronator and the brachialis muscles, a region with little interposed fat. It becomes easier to identify more distally as it courses between the superficial and deep heads of the pronator teres and reaches the level of the lacertus fibrosus.
Nerve Lesion Classification and Imaging Characterization
Nerve fibers are composed of axons and their supporting Schwann cells. The nerve fibers are surrounded by loose connective tissue called the endoneurium. The nerve fibers and surrounding endoneurium are grouped together in bundles referred to as fascicles. The fascicles are surrounded by a layer of collagenous connective tissue called the perineurium. The number of fascicles present in a particular nerve determines its size (15–17).
Nerve injuries may be the result of mechanical and functional compression, repetitive friction, traction, and traumatic disruption. Several classification systems have been used to describe nerve injuries. Seddon (18) reported three grades of injury based on severity: neurapraxia, axonotmesis, and neurotmesis. Neurapraxia, associated with nerve compression and loss of nerve conduction, is the least severe with the greatest potential for reversibility. The injury, in this case, is limited to the myelin sheath without axonal disruption. Axonotmesis
is characterized by axonal disruption without damage to the Schwann cells and endoneurium, resulting in Wallerian degeneration. Axonal regeneration is possible. The most severe injury is that of neurotmesis in which axonal disruption and discontinuity of the surrounding connective tissues is present with no chance of regeneration.
is characterized by axonal disruption without damage to the Schwann cells and endoneurium, resulting in Wallerian degeneration. Axonal regeneration is possible. The most severe injury is that of neurotmesis in which axonal disruption and discontinuity of the surrounding connective tissues is present with no chance of regeneration.
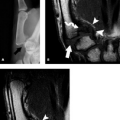
Although MRI can diagnose severe posttraumatic nerve injuries by identifying morphologic findings that include discontinuity of the nerve and a lax coiled spring appearance in cases of transection, it is less specific in distinguishing between lesser grades of injury. In general, both changes in morphology and signal intensity within peripheral nerves are nonspecific findings. Enlargement of a nerve in continuity may be observed proximal or distal to a site of compression or previous injury. A nerve may also appear flattened on MRI at a site of entrapment or subluxation. An increase in signal intensity on T2-weighted images is the hallmark of most neuropathies on MRI but offers no specific information to discern etiology or distinguish between low-grade nerve injuries (Fig. 13.7). Moreover, the absence of morphologic or signal intensity change in a nerve does not guarantee structural and functional integrity (19, 20). The role of intravenous contrast material administration in conjunction with MRI remains controversial in the assessment of suspected nerve pathology. Although some studies suggest that nerve enhancement reflects edema, and the lack thereof can be seen with ischemia, other studies showed poor correlation with enhancement and clinical manifestations of nerve disorders (21–23).
MRI can also be used to identify the end-organ manifestation of nerve pathology. MRI may demonstrate signal abnormality in denervated muscle (24). Early denervation manifests as high signal intensity on fluid-sensitive sequences with normal signal intensity on T1-weighted sequences. The signal-intensity changes correlate with the degree of nerve injury. In the neuropraxic type, the signal intensity within the muscle is normal, whereas in the axonotmetic and neurotmetic types of injury, there is increased signal within the muscle. In the setting of chronic denervation, loss of muscle volume and/or fatty infiltration of muscle can be seen. This is best identified on T1-weighted images. The MRI findings of signal and morphology change in the muscle, like in the nerve, is nonspecific and can be encountered in a variety of disease processes, including inflammatory or infectious processes, rhabdomyolysis, compartment syndrome, or neoplastic involvement. It is the distribution of muscle involvement in such cases, however, that can lead to nerve pathology as a potential source of MRI findings. The distribution of abnormal muscles on MRI may lead to a deduction about the specific nerve that may be injured and prompt a careful search along the course of that nerve for an abnormality. In addition, in posttraumatic muscle injury, there is typically perifascial edema that is not commonly encountered in the setting of denervation.
Nerve Entrapment Disorders
The nerves about the elbow are susceptible to various compressive neuropathies at both the level of the articulation and the proximal forearm. As previously noted, the diagnosis is based on some combination of clinical history, physical examination findings, electrodiagnostic studies, and imaging findings. The following discussion reviews some of the more commonly encountered entrapment disorders about the elbow.
Ulnar Neuropathy at the Elbow
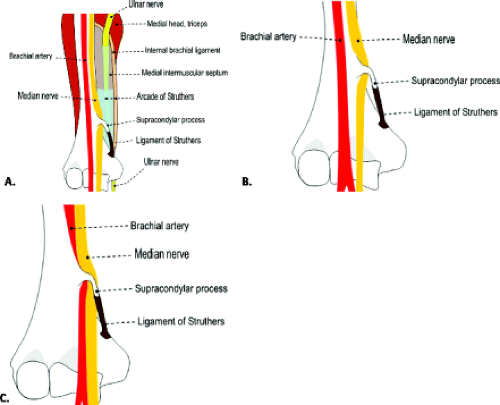
Ulnar neuropathy at the elbow is the most common neuropathy at the elbow and the second most common neuropathy in the upper extremity after carpal tunnel syndrome (25). There are a few potential compression sites along the course of the ulnar nerve at the elbow and along the proximal forearm, the most common of which is the cubital tunnel. Most proximally, however, the Arcade of Struthers and the edge of the medial intermuscular septum produces the first potential point of ulnar nerve entrapment. The Arcade of Struthers, not to be confused with the ligament of Struthers and
supracondylar process, is formed by the internal brachial ligament. This ligament extends from the medial head of the triceps in the lower third of the humerus to the humeral shaft, forming an arcade for passage of the ulnar nerve. One study found this arcade to be present in 14 of 20 cadaveric specimens (26). Less frequent sites of compression include Osborne’s fascia, a fibrous band that connects the proximal edge of the flexor carpi ulnaris muscle to the medial epicondyle and the deep flexor pronator aponeurosis (9).
supracondylar process, is formed by the internal brachial ligament. This ligament extends from the medial head of the triceps in the lower third of the humerus to the humeral shaft, forming an arcade for passage of the ulnar nerve. One study found this arcade to be present in 14 of 20 cadaveric specimens (26). Less frequent sites of compression include Osborne’s fascia, a fibrous band that connects the proximal edge of the flexor carpi ulnaris muscle to the medial epicondyle and the deep flexor pronator aponeurosis (9).
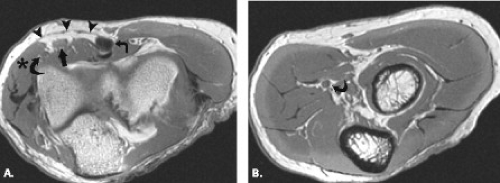
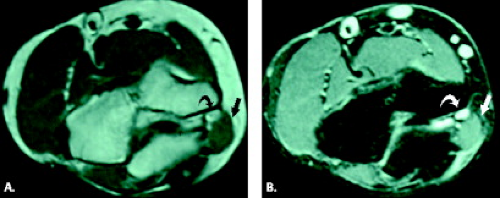
Cubital tunnel syndrome results from narrowing of the cubital tunnel or repetitive stress and traction on the ulnar nerve. It can be classified as physiological, acute, and subacute external compression as well as chronic compression. The pathoanatomy of cubital tunnel syndrome is predicated on diminished canal volume, increased intraneural pressure, and ulnar nerve elongation and excursion (13). In normal subjects, the arcuate ligament becomes taut and more closely apposed to the floor of the cubital tunnel with flexion of the elbow. The cross-sectional area of the tunnel decreases with subsequent elevation of intraneural pressure resulting in physiological nerve compression (14). This fact likely accounts for the occurrence of the syndrome in certain occupations prone to repetitive elbow flexion such as carpenters, painters, seamstresses, and musicians at which point repetitive physiological compression may become symptomatic. Chronic compression may be secondary to mass lesions, including bursae, ganglia, inflammatory synovitis, posttraumatic degenerative changes, ectopic calcifications, or ossification (Fig. 13.8) (27, 28). Anomalous anatomic structures such as the anconeus epitrochlearis may cause chronic nerve compression as well as, in rare cases, the ligament of Struthers, normally associated with median nerve compression (Fig. 13.9) (1, 29).
The classic clinical expression of cubital tunnel syndrome is numbness of the fifth digit, gradual development of hand weakness, atrophy of the first dorsal interosseous muscle, and reduced grip strength of the
deep fifth digit flexor and flexor carpi ulnaris muscles. In addition, the patient may experience radiating pain with elbow flexion and manual compression in the region of the cubital tunnel.
deep fifth digit flexor and flexor carpi ulnaris muscles. In addition, the patient may experience radiating pain with elbow flexion and manual compression in the region of the cubital tunnel.
The etiology of cubital tunnel syndrome can be somewhat elusive, with one study demonstrating 23% of cases with no demonstrable cause (30). In other studies, however, operative findings in patients with clinically diagnosed cubital tunnel syndrome have included morphology changes in the nerve, including both attenuation and thickening, fibrous nodules along the nerve, fibrous bands causing nerve constriction, adhesions within the tunnel blocking nerve movement, ganglion formation as well as the presence of an anomalous muscle (anconeus epitrochlearis) in the minority of cases (28). Coexistent osteoarthritis of the elbow has been reported in greater than 30% of patients with cubital tunnel syndrome (27).
Clinically diagnosed ulnar neuropathy often presents with increased signal intensity on fluid-sensitive sequences and appears to represent a sensitive and specific method for the diagnosis of cubital tunnel syndrome. One study reported signal changes on MRI in 97% of patients with a clinical diagnosis of cubital tunnel syndrome as compared with a 77% positive diagnosis with electrodiagnostic methods (31). The signal alteration may be better appreciated in the ulnar nerve as a result of its relatively large size. MRI detection of fibrous bands, morphology changes, masses, secondary degenerative changes at the elbow causing nerve compression as well as detection of anomalous muscle identification can be detected by MRI (31–33).
In addition to cubital tunnel syndrome, excessive mobility of the ulnar nerve may result in traction neuritis. Because the ulnar nerve may sublux in 10% to 16% of healthy subjects, clinical correlation regarding the presence of ulnar nerve symptoms is necessary (13, 34). The nerve may subluxate to the posteromedial margin of the cubital tunnel or frankly dislocate and be found anteromedial to the medial epicondyle. Ulnar nerve dislocation may be secondary to congenital absence, laxity or a tear of the arcuate ligament, a hypoplastic trochlea, or posttraumatic cubitus valgus deformity. Ulnar nerve dislocation may also be associated with snapping triceps syndrome (35, 36). Ulnar nerve subluxation and dislocation can be seen on routine axial MR images, but elbow flexion may allow better visualization of displacement.
Posterior Interosseous Nerve Syndrome
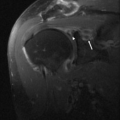
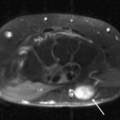
Posterior interosseous nerve syndrome, also known as supinator entrapment syndrome, is an entrapment of the deep branch of the radial nerve just distal to the elbow, which can result in paresis or paralysis of the digital and thumb extensor muscles. There are five potential compression sites, the most common of which is at the tendinous proximal edge of the supinator muscle in the region of the Arcade of Frohse. Other less common sites of radial nerve compression are at the anterior capsule of the radiocapitellar joint, in the region of the Leash of Henry, small recurrent vessels that cross the posterior interosseous nerve, at the fibrous edge of the ECRB, and at the distal margin of the supinator muscle, where the posterior interosseous nerve exits the supinator tunnel.
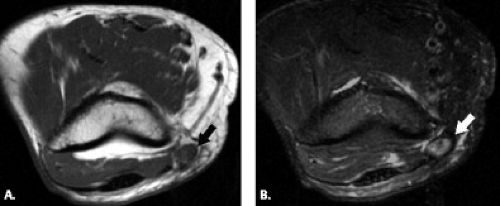
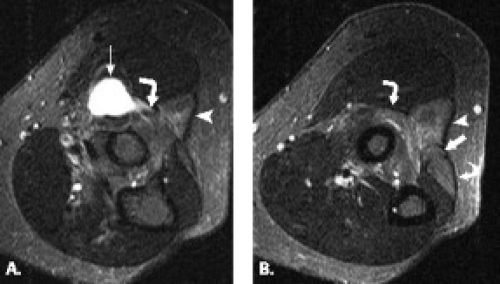
A variety of conditions may cause posterior interosseous nerve palsy. These include trauma, extra-articular tumors, or tumor-like masses such as ganglia, lipoma, or distended bursae (Fig. 13.10) (37–40). Intra-articular lesions such as rheumatoid synovitis, synovial chondromatosis, intracapsular chondroma, synovial hemangioma, and pseudogout have been reported as well as tuberculous arthritis and septic arthritis of the elbow (41–48).
Posterior interosseous nerve syndrome manifests clinically as insidious onset of pain and weakness of the muscles it innervates without sensory dysfunction. The complete syndrome is characterized by loss of extension of all digits and by a decrease in the ability to dorsiflex the wrist. Partial involvement can also occur (9).
It is important to include the whole radial tunnel in the MRI study when posterior interosseous or radial tunnel syndromes are suspected. In cases of radial tunnel and posterior interosseous nerve syndromes, imaging
the patients with the forearm in pronation has been suggested as a result of the dynamic nature of compression associated with these syndromes. Familiarity with the course of the radial nerve as well as the likely locations for compression facilitates the detection of subtle abnormalities that could cause this entity. As noted previously, if the nerve can be identified, its morphology and signal intensity should be assessed. In addition, the muscle denervation pattern, if present, may indicate the level of injury. If the triceps, anconeus, brachioradialis, and extensor carpi radialis longus muscles are involved, the radial nerve is compromised before it divides into the posterior interosseous and superficial radial branches. In posterior interosseous nerve and radial tunnel syndromes, muscle edema or atrophy is most frequently seen in the supinator and extensor muscles.
the patients with the forearm in pronation has been suggested as a result of the dynamic nature of compression associated with these syndromes. Familiarity with the course of the radial nerve as well as the likely locations for compression facilitates the detection of subtle abnormalities that could cause this entity. As noted previously, if the nerve can be identified, its morphology and signal intensity should be assessed. In addition, the muscle denervation pattern, if present, may indicate the level of injury. If the triceps, anconeus, brachioradialis, and extensor carpi radialis longus muscles are involved, the radial nerve is compromised before it divides into the posterior interosseous and superficial radial branches. In posterior interosseous nerve and radial tunnel syndromes, muscle edema or atrophy is most frequently seen in the supinator and extensor muscles.
Radial Tunnel Syndrome
Radial tunnel syndrome is produced by radial nerve compression within the radial tunnel, a defined space that begins at the radiocapitellar joint and extends to the distal aspect of the supinator muscle. It is bounded medially by the brachialis muscle and laterally by the brachioradialis, extensor carpi radialis longus and brevis muscles. Potential sites of compression are the same as those encountered in posterior interosseous nerve syndrome and include the fibrous margin of the ECRB muscle, fibrous bands at the level of the radiocapitellar joint, the radial recurrent artery, the Arcade of Frohse proximally as the nerve passes distally through the supinator muscle, and a fibrous band at the distal margin of the supinator (49–51).
Pain is the most common primary presenting symptom in radial tunnel syndrome. Classically, there is no motor dysfunction (52). As a result of the lack of objective findings, there has been controversy as to the existence of this syndrome. It is the only nerve compression syndrome in which signs and symptoms are not based on the distribution of the nerve. A point of maximal tenderness is usually encountered over the anterior radial neck. Compression of the extensor muscle group may also cause pain as can active resistance to extension of the middle finger. Physical examination findings are exacerbated by placing the elbow in extension with the forearm pronated and the wrist flexed. Radial tunnel syndrome was first described in a patient with resistant tennis elbow, and an association between radial tunnel syndrome and the clinical entity of lateral epicondylitis has been noted, coexisting in up to 5% of patients (53–56).
Pronator Syndrome
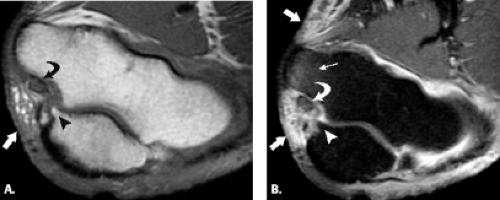
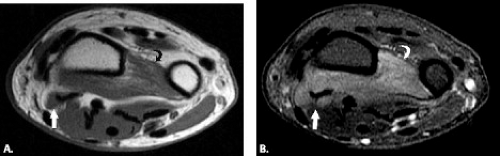
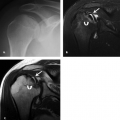
Pronator syndrome has been classically and most commonly described as the result of compression of the median nerve between the two heads of the pronator teres muscle. Other potential sites of compression include the flexor digitorum superficialis, the lacertus fibrosus, and the supracondylar process, from most to least common (Fig. 13.11) (49, 57–59). After the pronator muscle, the second most common site of median nerve compression is at the origin of the flexor digitorum superficialis muscle. In this region, approximately 2 cm distal to the ulnar head of the pronator teres muscle, a fibrous arch can be present that produces mass effect on the nerve (57, 60, 61). More proximally, the lacertus fibrosus arises from the distal bicipital tendon and inserts on the antebrachial fascia, tethering the tendon to the fascia of the pronator–flexor muscle group. If this structure becomes thickened, it can produce compression on the median nerve (58, 59). The most proximal site of compression is the supracondylar process of the humerus and associated ligament of Struthers. This osseous process, reported to be present in approximately 3% of individuals, arises from the anteromedial aspect of the distal humerus, 5 cm proximal to the medial epicondyle (49, 62, 63). In the absence of the osseous supracondylar process, the ligament of Struthers can replace it proximally while following its same course distally to the attachment on the medial epicondyle forming a fibro-osseous or fibrous canal through which the nerve must pass. In some cases, the nerve is accompanied by the brachial artery. This entity can also be called supracondylar process syndrome (49, 62, 63).
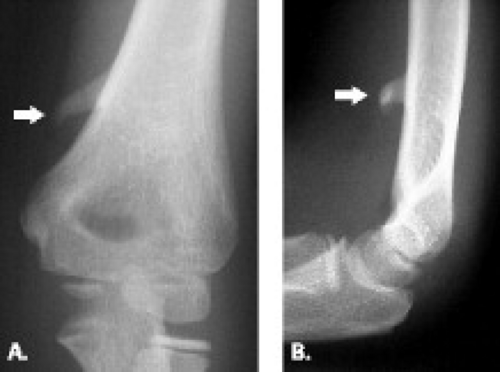 Figure 13.11 Supracondylar process. A: Frontal and B: lateral radiographs show a supracondylar process (arrow) emanating from the anteromedial aspect of the humerus. |
Additional causes of median nerve compression include the presence of anomalous muscles about the elbow, including Gantzer’s muscle, an anomalous flexor pollicis longus, the palmaris profundus muscle, and a flexor carpi radialis brevis muscle (64, 65). Anomalous vessels such as an aberrant radial artery has been implicated in median nerve compression (64). Median nerve entrapment after closed reduction of an elbow dislocation has been reported (66). An enlarged bicipitoradial bursa may also result in mass effect on the median nerve.
Pronator syndrome commonly occurs with strenuous activities such as weightlifting and in occupations requiring repetitive pronation of the forearm with the elbow extended. It can be confused with carpal tunnel syndrome, because both may cause numbness and paresthesias in the median nerve innervated digits, weakness of the thenar muscles, and pain in the wrist and forearm. It differs from carpal tunnel syndrome in that it does not produce nocturnal symptoms. In addition, dysesthesias are encountered in the palmar triangle or in the skin overlying the thenar eminence in pronator
syndrome. These regions are innervated by the palmar cutaneous branch of the median nerve, a branch that originates proximal to the transverse carpal ligament.
syndrome. These regions are innervated by the palmar cutaneous branch of the median nerve, a branch that originates proximal to the transverse carpal ligament.
Like in the MRI evaluation of any suspected nerve pathology, it is essential to include all potential sites of involvement in the field of imaging. In the case of the median nerve, this includes the distal third of the arm to the flexor digitorum superificialis muscle in the proximal forearm. As previously noted, primary nerve lesions, evidence of acute or chronic nerve pathology in the form of signal alteration or morphology change as well as structures causing extrinsic mass effect on the nerve must be sought out. In the case of pronator syndrome, signal abnormalities or atrophy in the innervated muscle groups of the median nerve distribution would include the pronator teres, the flexor carpi radialis, the palmaris longus, and the flexor digitorum superficialis muscles. Anterior interosseous nerve denervation would depict edema or atrophy of the radial half of the flexor digitorum profundus, the flexor pollicis longus, and the pronator quadratus muscles (9).
Anterior Interosseous Nerve Syndrome
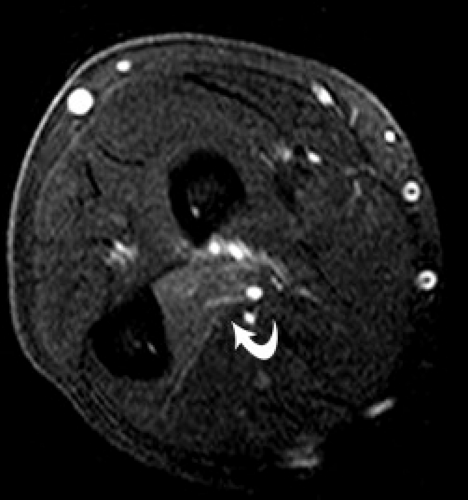
Anterior interosseous nerve syndrome was first described by Tinel in 1918 and was further delineated by Kiloh and Nevin in 1952 (Figs. 13.12 and 13.13) (49). Compression sites of the anterior interosseous nerve include the ulnar head of the pronator teres muscle, the flexor digitorum superficialis, accessory muscles, anomalous arteries, and fibrous bands (57, 65). When the syndrome is complete, denervated muscles include the flexor pollicis longus, the two radial profundus tendons, and the pronator quadratus. No sensory changes occur, distinguishing this entity from both pronator and carpal tunnel syndromes. The symptoms reveal weakness of the thumb and index finger with a disturbance of the pinch mechanism. Anterior interosseous nerve syndrome can simulate and should be distinguished from flexor tendon rupture (67).
MRI of Elbow Synovium
Synovial membranes line the diarthrodial (movable) joints, bursae, and tendon sheaths of the body. The primary function of this specialized, vascular tissue is to serve as a filter system that lubricates and nourishes the articular structures as well as serving as a shock absorber. The synovium is affected by a variety of disorders that can be localized to a specific articulation such as the elbow or can be systemic in nature. These include inflammatory, infectious, degenerative, traumatic, or neoplastic categories of disease. MRI, with its excellent soft tissue contrast and multiplanar imaging capabilities, has revolutionized the diagnostic capabilities within the musculoskeletal system and provided a
noninvasive means to study both the anatomy and pathology in the synovium.
noninvasive means to study both the anatomy and pathology in the synovium.
Technical Considerations
Although it is widely accepted that MRI has revolutionized the ability to visualize and characterize delicate soft tissue structures such as the synovium, debate still exists regarding the optimal imaging technique for its evaluation. Much of this debate focuses on the use of intravenous gadolinium for synovial evaluation.
Standard MRI
Articulations of the synovial type are comprised of the ends of bones that are linked together by a fibrous capsule and frequently by accessory ligaments that are located either within that capsule or outside of it. The fibrous capsule encloses the joint completely in most cases and is lined throughout by synovial membrane. This synovial membrane, in general, extends on to all intra-articular surfaces except those actually involved in compression contact during joint activity. The fibrous joint capsule can be seen as a thin, low-signal–intensity linear structure on MRI on both T1- and fluid-sensitive sequences. The delicate, vascular inner synovial membrane is so thin it is difficult to distinguish from its outer fibrous capsule in most cases. However, when visible, it is intermediate in signal intensity on proton density- or T1-weighted sequences and high in signal intensity on fluid-sensitive sequences, paralleling that of simple joint fluid.
Although it is generally accepted that the use of intravenous contrast administration in conjunction with MRI portrays the synovium to best advantage, the synovium can certainly be assessed with standard MRI sequences. Like with all structural evaluation by MRI, morphology and signal intensity are the general tools used to establish the presence of pathology. In the case of the synovium, thickening or morphology changes, including frond-like densities or nodularity, reflect inflammation and are generally best seen on T2-weighted sequences. Several investigators have also described areas of synovial thickening to have intermediate to high signal intensity on proton density- or T1-weighted images as compared with adjacent joint fluid (68–71). In some cases, the thickened synovium of synovitis showed intermediate signal intensity on T2-weighted images as compared with the high signal intensity of adjacent simple joint fluid, and in other cases, the presence of synovitis could not be distinguished from joint fluid with unenhanced MRI of the knee (72). It is for this reason that MRI in conjunction with the intravenous administration of gadolinium is often used for the synovial evaluation.
Intravenous Administration of Gadolinium
The few studies that document the imaging appearance of the synovium in an asymptomatic patient population report discrepant results with regard to presence and degree of synovial enhancement after intravenous contrast administration (71, 73–78). In a study by Boegard et al. (73) designed to evaluate the occurrence and extent of enhancement of synovial structures after the intravenous administration of a gadolinium-based contrast material in the knees of middle-aged, healthy, asymptomatic individuals, synovial enhancement was present in each articulation. Moreover, the thickness of synovial structures within these asymptomatic articulations showed significant variability with the exception of the suprapatellar recess, in which synovial thickness was constant and minimal. This study emphasizes the difficulty in identifying a gold standard in synovial assessment and establishes that synovial enhancement can occur in the asymptomatic articulation.
In the setting of acute synovial inflammatory processes, the majority of studies contend that MRI in conjunction with the administration of intravenous gadolinium-based contrast material is beneficial in distinguishing synovial proliferation from adjacent joint fluid because both appear low in signal intensity on T1-weighted and high in signal intensity on T2-weighted, unenhanced images (69–71, 74–83). Some authors have argued against the usefulness of contrast-enhanced imaging for synovial evaluation, noting that the exact border between effusion and synovium can also be difficult to distinguish after the administration of intravenous contrast. This is the result of the diffusion of contrast material into the joint over time, resulting in enhancement of the effusion (76, 84). It appears, however, that the signal intensity of inflamed synovial tissue on T1-weighted MR images increases markedly within the first minute after intravenous administration of contrast (84–86). On the contrary, the rate of signal increase in effusion is slower, beginning in the periphery where contrast material diffuses into the joint space from the synovium reaching maximum signal intensity 15 minutes after intravenous injection (76). Based on the temporal relationship of synovial versus effusion enhancement, volumes of both joint fluid and synovium can be determined in a reasonably accurate fashion with a maximal analytical error of 20% (10, 84, 86, 87).
MRI in conjunction with the intravenous administration of gadolinium-based contrast material has also proven helpful in characterizing disease activity in synovial inflammation. As synovial inflammation becomes inactive, a gradual decrease in vascularization occurs with transformation to fibrous tissue (74).
Gradient-echo Imaging Sequences
The use of gradient-echo imaging sequences is particularly useful in synovial disorders in which hemosiderin deposition predominates. The paramagnetic effect of hemosiderin results in low signal intensity on all MR sequences. Signal dropout, however, is most obvious on heavily T2*-weighted gradient-echo images and is enhanced even further by high field strength (88).
Capsular and Bursal Anatomy
The joint capsule invests the three articulations of the elbow forming a single contiguous joint space. The capsule is comprised of two layers: a deep synovial lining and a more superficial fibrous layer. Three major fat pads are situated between the synovial and deep fibrous
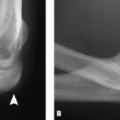
layers, making their location intra-articular but extrasynovial. Two anterior fat pads correspond to the capitellar and trochlear fossae, whereas a single posterior fat pad is concealed within the olecranon fossa. Therefore, in the presence of a joint effusion confined by the synovial capsule, the extrasynovial fat pads are elevated resulting in the radiographic signs of a visible posterior fat pad and an elevated anterior fat pad, the “anterior sail” sign (Fig. 13.14).
layers, making their location intra-articular but extrasynovial. Two anterior fat pads correspond to the capitellar and trochlear fossae, whereas a single posterior fat pad is concealed within the olecranon fossa. Therefore, in the presence of a joint effusion confined by the synovial capsule, the extrasynovial fat pads are elevated resulting in the radiographic signs of a visible posterior fat pad and an elevated anterior fat pad, the “anterior sail” sign (Fig. 13.14).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree