Sequence
Brief description
SSFP (Steady-state free precession)
Excellent tissue contrast between cardiac structures and blood pool in a time-resolved cine imaging sequence Useful for measuring chamber volumes, function and mass. Accurate measurement of aortic valve annulus for TAVR sizing
T2 STIR
T2-weighted sequence with short tau inversion recovery
Myocardial water content or edema associated with acute injury will appear brighter than normal myocardium
MOLLI T1 mapping (modified look-locker inversion recovery)
Novel pulse sequence to evaluate for diffuse fibrosis as a predictor of recovery and/or prognosis
T1-sensitive inversion recovery (Late gadolinium enhancement)
Detection of focal myocardial scar for risk statisfication of patients with aortic stenosis
Detection of myocardial infarction to assess patients pre-transapical TAVR
Phase-contrast velocity mapping
Flow imaging and quantification Measurement of valvular and vascular mean and peak velocities and regurgitant flow
Cardiovascular Magnetic Resonance Imaging for Aortic Stenosis
Imaging plays a central role in establishing the diagnosis of aortic stenosis and also helping to define the etiology of the valvular pathology. The three primary causes of valvular aortic stenosis are calcific disease of a trileaflet valve, abnormal bicuspid (or unicuspid valve) complicated by calcification, and rheumatic valve disease. Beyond establishing the precise etiology, noninvasive imaging is essential for determining disease severity, impact on cardiac morphology and function, and planning of medical or interventional therapies.
While echocardiography has been the stalwart of noninvasive assessment of aortic stenosis, the most recent appropriate use criteria proposed by the American College of Cardiology Foundation consider the performance CMR to characterize native and prosthetic cardiac valves—including short-axis planimetry of stenotic disease and quantification of regurgitant disease—as appropriate in patients with technically limited echocardiographic images [3, 4]. In addition, CMR is considered appropriate to evaluate morphology and function when information from other testing is discordant and to evaluate the contribution of a non-valvular etiology—typically with gadolinium-based tissue characterization—when mixed disease is suspected.
In our experience at Institut universitaire de cardiologie et de pneumologie de Québec of 2,500 CMR studies performed yearly, the proportion of clinical indications for assessment of valvular disease is approximately 10 %. Contraindications to CMR rarely prevent a study (<2 %) and usually consist of a non-MR compatible pacemaker or defibrillator or ferromagnetic material in or near to the central nervous system, eyes, or inner ears. Importantly, other ferromagnetic material—including sternotomy wires and most implanted heart valves and device—is not contraindicated for MR imaging (refer to www.MRIsafety.com for complete list).
While standard CMR assessment of valvular heart disease is typically performed with contrast, a robust CMR examination may be performed without gadolinium contrast if contraindications exist, such as is the case in severe kidney failure (creatinine clearance <30 cc/min). As previously stated, one important strength of CMR is its ability to perform 3D cine imaging (morphology and function) and flow measurement noninvasively and without contrast agents. In our own institutional experience, approximately 25 % of individuals undergoing TAVR have severe kidney failure. For these patients, contrast-free CMR provides all the necessary information regarding etiology and severity of valvular aortic stenosis, its consequences and concomitant disease, and morphology necessary for the planning of TAVR.
Cardiac Morphology and Function
The usefulness of CMR for cardiac morphology and function has been well described elsewhere [5]. For patients with aortic valve stenosis, an accurate measurement of left ventricular (LV) volumes, mass, and function using full 3D coverage is important to help guide therapeutic intervention and provide important prognostic information (Fig. 14.1). 3D techniques are particularly useful for the accurate measurement of ventricular volume, function, and mass when there is regional remodeling where geometrical assumptions from 2D imaging are less accurate and reliable [6]. Beyond measuring these parameters at a single time point, serial assessment of cardiac structure, morphology, and function is highly reproducible by CMR and plays a vital role in determining whether valvular disease is manifesting with progressive deterioration. The excellent spatial and contrast resolution afforded by modern bright blood sequences allows for reproducible and accurate functional analysis that has been shown to be superior to echocardiography [7, 8]. CMR has also proven helpful in accurately assessing reverse remodeling following aortic valve replacement for aortic stenosis [8–10].
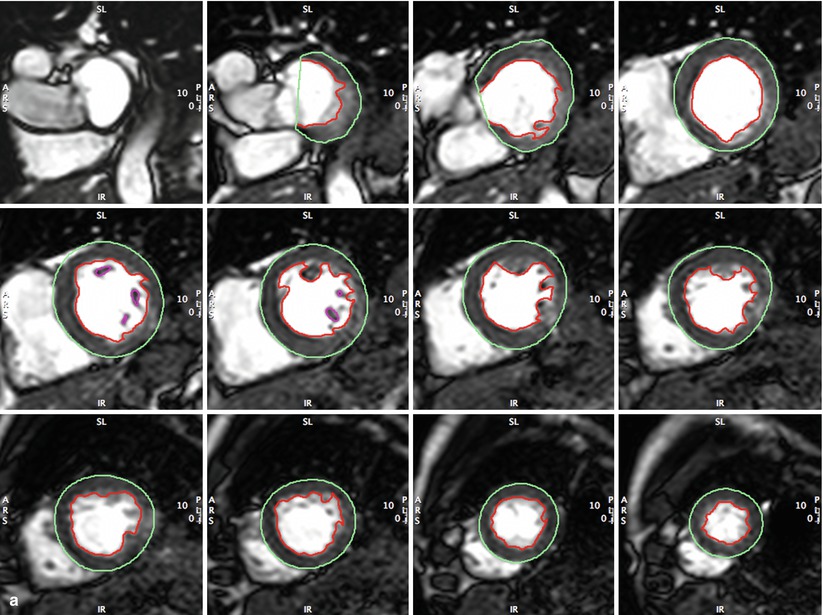
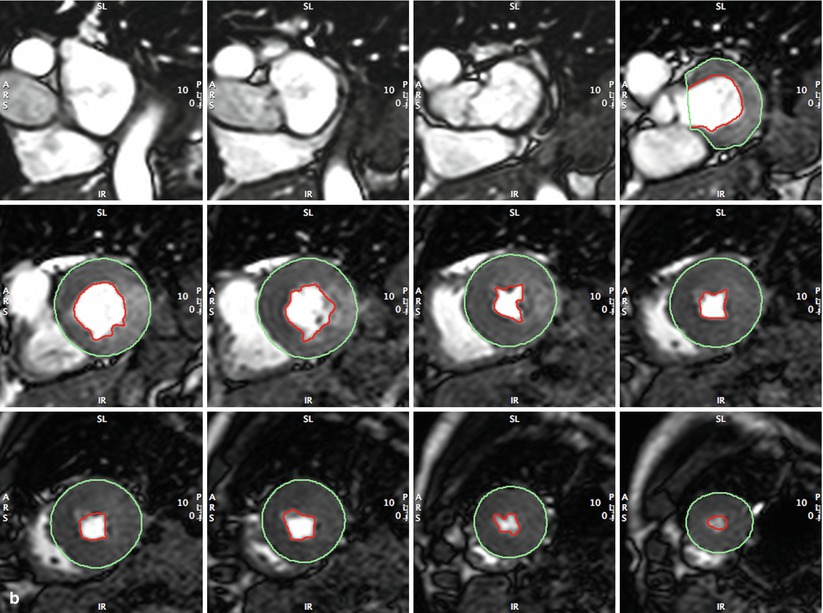


Fig. 14.1
(a, b) Full coverage of left ventricle in short axis by standard steady-state free precession (SSFP) CMR. This approach is typically used at 20–30 phases per cardiac cycle for accurate and reproducible measurement of left ventricular end-diastolic (Panel a) and end-systolic (Panel b) volumes, ejection fraction, and left ventricular mass (LVEDV, LVESV, LV mass, LVEF). Cardiac morphology and function may thus be followed serially to detect disease progression/regression without ionizing radiation
Valvular Morphology and Function
Cine SSFP imaging allows the dynamic visualization of the aortic valve throughout the cardiac cycle without the need for contrast or ionizing radiation. Importantly, the aortic valve can be reformatted in any desired plane. Typically, the valve is imaged in two orthogonal long-axis views displaying the left ventricular outflow tract and aortic root and in a short-axis view following the plane of the aortic annulus and left ventricular outflow tract. Despite respiratory standstill, cardiac motion leads the aortic valve plane to temporarily migrate out of the imaging plane during the cardiac cycle. Motion correction software is required to remain in the annular plane throughout the cycle.
Dynamic imaging in short axis is also useful to first confirm the tricuspid or bicuspid nature of the aortic valve. Following that, the aortic valve area is planimetered to assess the severity of aortic stenosis (Fig. 14.2). Planimetered measurements of the aortic valve by CMR have been shown to correlate highly with measurements of AVA by transesophageal echocardiography (TEE) and retrospective ECG-gated multidetector CT [11–13]. Correlation with TEE is strongest with cine SSFP, as compared to gradient-echo sequences by CMR [14]. These same pulse sequences and image planes can be used to assess the aortic root dimensions to provide additional important information to guide surgical or percutaneous intervention [15].
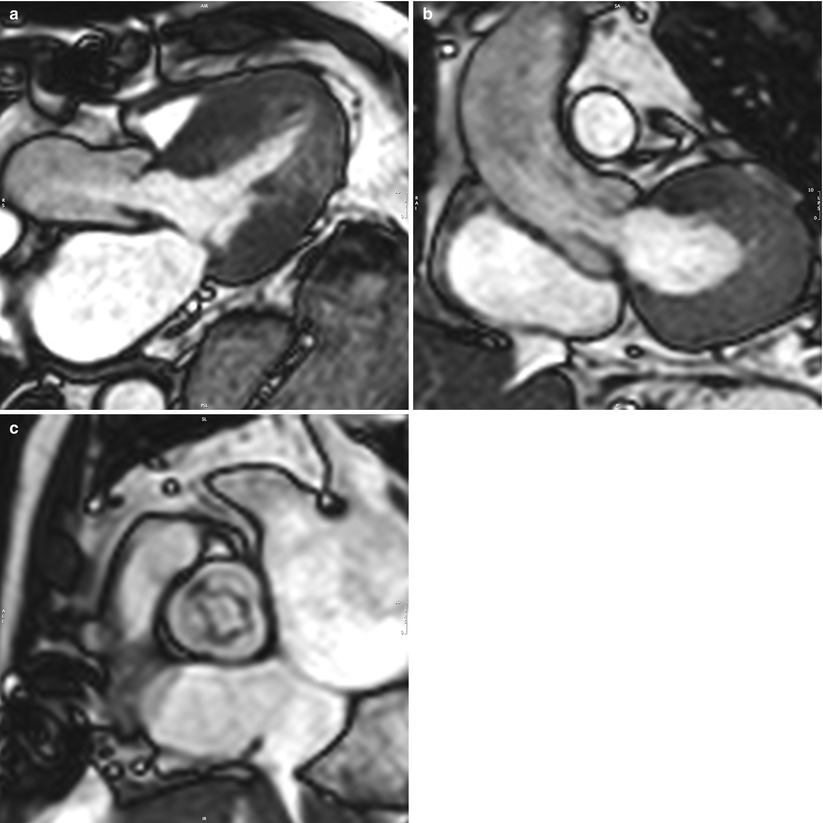

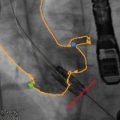
Fig. 14.2
(a–c) Cine SSFP CMR of the aortic valve, left ventricular outflow tract, and aortic root in two orthogonal long-axis planes (Panels a and b) and the short-axis plane (Panel c). Dynamic 3D imaging allows radiation-free and contrast-free measurement of the aortic valve opening and annulus surfaces by planimetry, as well as the annulus diameters in any given plane
Historically, morphological imaging of valvular function has proven very useful in establishing the underlying mechanism of disease but has been a poor substitute for the hemodynamic measurement of the severity of stenosis and regurgitation. While a true planimetered valve area provides excellent anatomical analysis of the aortic valve, it has been previously shown that the physiological effects, as measured by effective orifice area (EOA), outweigh the anatomical assessment—calculated as an anatomic aortic valve area (AVA)—in determining disease severity and its impact [16]. To overcome the limitations of anatomical valvular imaging with bright blood cine sequences, phase-contrast velocity mapping (PC-CMR) measures the velocity of blood flow in any given plane, allowing the precise measurement of mean and maximum velocities of the entire blood pool flowing through the vessel of interest and avoiding the dependence on interrogation angles and beam position seen with Doppler echocardiography. This method has been validated against the clinical standard Doppler echocardiography for measurement of flow velocities [17, 18] and the physiological EOA in mild to severe AS [19]. Calculating AVA by the continuity equation provides similar results whether using CMR or TTE [20]. Ongoing research aims to further refine PC-CMR in high-velocity turbulent jets of severe AS where PC-CMR has been known to underestimate peak gradients [21]. By imaging the short-axis plane immediately above the plane of the aortic valve annulus, forward and regurgitant flow is measured completely in order to determine precise regurgitant fraction, which may be underestimated by echocardiography following TAVR (Fig. 14.3) [22].
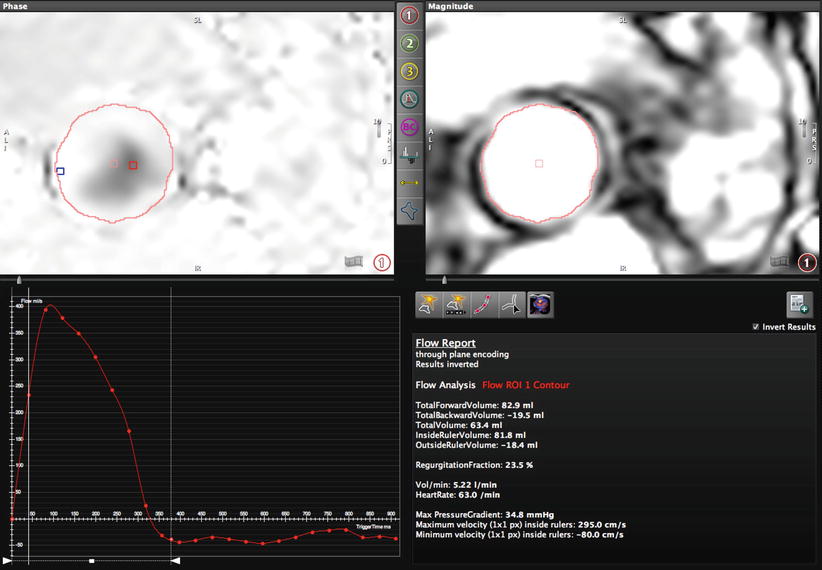

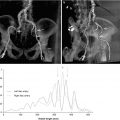
Fig. 14.3
Phase-contrast (PC-CMR) performed in the aortic root above the valve in the plane of the annulus (Fig. 14.4). Through-plane encoding measurements include a maximum velocity of 295 cm/s (maximum pressure gradient of 34.8 mmHg), total forward volume of 82.9 ml, and backward volume of 19.5 ml for a regurgitant fraction of 23.5 %. Such PC-CMR measurements do not suffer from Doppler beam angle dependency or errors brought on by multiple jets
Myocardial Tissue Characterization
Severe aortic valve disease is associated with alterations in myocyte structure that can result in progressive myocardial fibrosis. Preliminary data has suggested that greater myocardial fibrosis measured by CMR may impede the improvement in LV ejection fraction following surgical valve replacement [23]. The measurement of myocardial fibrosis by CMR relies on proton relaxation characteristics. The T1 or spin–lattice relaxation time corresponds to the specific time decay constant when the proton recovers approximately 63 % of its longitudinal magnetization equilibrium value. The T1 relaxation time depends on the molecular environment of the water molecules in the tissue and therefore characterizes each tissue very specifically. T1 relaxation times vary significantly from one type of tissue to another, but also within the same tissue depending on its physiopathological status (inflammation, edema, or fibrosis). While focal fibrosis such as that incurred by ischemic cardiomyopathy may be measured by LGE as described above, diffuse fibrosis may be measured by a variety of other CMR techniques, including contrast-enhanced T1 mapping, modified look-locker inversion-recovery (MOLLI) T1 mapping, and equilibrium-contrast CMR [24]. Each method carries strengths and limitations with respect to feasibility in the clinical setting.
Focal fibrosis measured by LGE appears to be common in the setting of significant myocardial hypertrophy with wall thickness in excess of 18 mm [25, 26]. When identified, this appears to be a truly limited process commonly involving less than 5 % of the total myocardial mass. While limited in scope, the presence of LGE has important prognostic significance with a fivefold increased hazard for all-cause mortality as compared to those without delayed enhancement with comparable severity of aortic stenosis [27]. Greater fibrosis on CMR is also known to be present in low-flow severe AS, a subpopulation of AS with poorer prognosis [28]. It has also been shown that focal fibrosis on LGE CMR is an irreversible finding that does not resolve with aortic valve replacement and elimination of the afterload disturbance [29].
Magnetic resonance spectroscopy has proven useful to study myocardial high-energy phosphate (HEP) metabolism in patients. Similar to that observed with dilated cardiomyopathy, significant AS may be associated with altered myocardial HEP metabolism, which may improve following corrective surgery [30–32]. This metabolic improvement appears to occur even in the presence of patient-prosthesis mismatch [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree