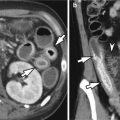
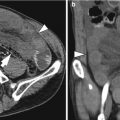
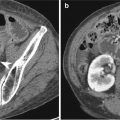
Pathologic T stage on AJCC 7th edition
CT criteria
T1
Tumor invades mucosa and submucosa
Focal thickening with enhancement in inner and/or middle layer; wall thickening and enhancement of inner layer with visible low-attenuation outer layer and clear fat plane around tumor; enhancement without thickening in gastric wall
T1a
Tumor invades lamina propria or muscularis mucosae
Not depicted on CT or linear mucosal enhancement without thickening
T1b
Tumor invades submucosa
Enhancing mucosal thickening with preservation of middle low-attenuation layer
T2
Tumor invades muscularis propria
Focal or diffuse wall thickening with enhancement and transmural involvement and has smooth outer wall border and clear fat plane around tumor
T3
Tumor penetrates subserosal connective tissue without invasion of viscera peritoneum or adjacent structures
T4a
Tumor invades visceral peritoneum (serosa)
Transmural thickening and enhancement with irregular and nodular outer border and/or perigastric fat stranding or haziness; hyperattenuating serosal layer
T4b
Tumor invades adjacent structures
Indistinct/irregular interface or direct tumor infiltration into adjacent organs
The accuracy of MDCT for N stage ranges from 60 to 80 % because about half of metastatic nodes have smaller than 5 mm size and reactive inflammatory change can enlarge the lymph node without metastasis. The criteria for metastatic lymph node on CT are any nodes 6–8 mm or greater in short-axis diameter, a cluster of three or more perilesional nodes or lymph nodes with strong enhancement (Lee et al. 2010).
Hematogenous metastases from gastric carcinoma most frequently involve the liver via portal venous drainage. AGCs can demonstrate peritoneal seeding of malignant tumor cells and CT findings for peritoneal metastasis include the presence of ascites with peritoneal thickening and/or enhancement, peritoneal nodule or plaques, omental and mesenteric fat stranding or nodular infiltration, and small bowel wall thickening and nodularity. Although the pooled sensitivity and specificity for CT in detecting liver metastasis are 0.74 (95 % confidence interval, 0.59–0.85) and 0.99 (95 % confidence interval, 0.97–1.00), respectively, the pooled sensitivity for CT in detecting peritoneal metastasis is very low (0.33; 95 % confidence interval, 0.16–0.56) in meta-analysis (Wang and Chen 2011).
6.2.4 Specific Types of Gastric Cancer
6.2.4.1 Gastric Mucinous Adenocarcinoma
Mucinous adenocarcinoma accounts for 10 % of gastric carcinoma. Histologically, it is characterized by extracellular mucinous pools which constitute at least 50 % of tumor volume. The tumor cells can form glandular architecture and irregular cell clusters, with scattered signet ring cells floating in the mucinous pools. The mucinous tumors have the worse prognosis mainly due to advanced stage at diagnosis. On CT, mucinous adenocarcinomas appear as fungating or diffusely infiltrative tumor with thickened low-attenuating middle or outer layer, corresponding to mucin pool located in submucosa or deeper layer. Some mucinous tumors may show punctate or miliary calcifications in the thickened gastric wall (Park et al. 2002).
6.2.4.2 Epstein-Barr Virus-Associated Lymphoepithelioma-Like Gastric Carcinoma
The lymphoepithelioma-like gastric cancer that is one of Epstein-Barr virus (EBV)-associated gastric cancers is pathologically characterized by dense lymphoid stroma and small nests of cancer cells and has a tendency to be present in upper part of the stomach. When this tumor invades into the submucosal layer, submucosal nodule composed of cancer cells with lymphoid stroma can be misdiagnosed as submucosal tumors like gastrointestinal stromal tumor or carcinoid tumor. CT images show ovoid enhancing submucosal mass with intact overlying mucosa or central ulceration (Kim et al. 2010).
6.2.4.3 Gastric Hepatoid Adenocarcinoma
Hepatoid adenocarcinoma is one of extrahepatic adenocarcinoma that shows hepatoid differentiation histologically and can produce α-fetoprotein. Although this type tumor can occur in lung, pancreas, esophagus, and colon, the stomach is the most frequently affected organ and gastric hepatoid adenocarcinoma is associated with an advanced age, elevated serum α-fetoprotein level, and aggressive clinical behavior resulting in poor prognosis. Gastric hepatoid adenocarcinomas show the eccentric gastric wall thickening or mass with heterogeneous enhancement and frequent adjacent organ invasions to the liver or pancreas on CT images. Also, this type tumor shows a tendency to hepatic and nodal metastases and venous invasion around primary gastric tumor or hepatic metastatic masses (Lee et al. 2007).
6.3 Gastric Neuroendocrine Tumor (NET)
GI NET in which tumor cells have both phenotypic features of endocrine and neural cells and express markers such as chromogranin-A and synaptophysin can be categorized into neuroendocrine tumor grade 1 (well-differentiated endocrine tumor, carcinoid tumor), neuroendocrine tumor grade 2 (well-differentiated endocrine carcinoma), and neuroendocrine carcinoma grade 3, large-cell or small-cell type (poorly differentiated endocrine carcinoma/small-cell carcinoma) according to WHO 2010 classification based on tumor histopathology, proliferative activity, and other tumor characteristics including site of origin, size, infiltration/invasion into other organ, and metastasis (Rindi et al. 2010). Mainly due to increased application of endoscopy, the frequency of gastric NET has been rising to 41 % of all GI NETs. In most of patients with gastric NET, there are no specific symptoms related to gastric NETs because these tumors are usually detected incidentally. In contrast to small bowel NETs, typical carcinoid syndrome is very rare in patients with gastric NETs. Because these tumors can give early hepatic metastases and regional lymph node metastases even in small size, the adequate staging evaluation should be mandatory. Based on the presence of hypergastrinemia, well-differentiated gastric NETs can be categorized into type I, associated with hypergastrinemia, chronic atrophic gastritis, and enterochromaffin-like cell hyperplasia; type II, seen in the setting of hypergastrinemia due to multiple endocrine neoplasia type I (MEN-I) and Zollinger-Ellison syndrome; and type III, not associated with hypergastrinemia (sporadic type). Type I gastric NETs are the most common type (75–80 % of gastric NETs) and more common in women patients (two or three times more than men). They usually appear as multiple and small (usually less than 1 cm) polypoid lesions in gastric body and fundus area. Type I gastric NETs generally represent benign disease course and rarely metastasize to lymph nodes (2 % of patients with type I tumor). Type II gastric NETs are associated with MEN1 syndrome and tend to be larger (often more than 1 cm) and show more aggressive clinical behavior (local metastasis in up to 30 %). In patients with type II NETs, there are multiple polyps or masses in which ulceration is sometimes present in the thickened rugal folds and the gastric wall. Type III gastric NETs, that are sporadic tumor, generally appear as usually large (often more than 2 cm), solitary tumors that may show ulceration. These tumors have an aggressive clinical behavior with local invasion and early metastases, especially when the tumor size is greater than 3 cm (Christopoulos and Papavassiliou 2005; Johnson et al. 2010).
On UGIS, gastric carcinoid tumors appear as small (almost less than 2 cm) sessile polypoid lesion. These tumors are usually seen as the small enhancing polyp on CT. In patients who have the associated Zollinger-Ellison syndrome, diffuse rugal folds thickening and multiple polyps or masses can be seen. The differential diagnosis of gastric carcinoid tumor that presents as small enhancing polypoid lesion can include small gastrointestinal stromal tumor, glomus tumor, and heterotopic pancreas. For the gastric neuroendocrine carcinoma, stomach is the most frequent site of GI neuroendocrine carcinomas (up to 59 % of all GI neuroendocrine carcinoma). On CT, gastric neuroendocrine carcinomas typically show obvious and homogeneous enhancement on arterial phase and further enhancement on venous phase since these tumors have an abundant blood supply. When these tumors spread to the liver, hepatic metastatic lesions usually show a homogeneous enhancement (Johnson et al. 2010).
Summary
1.
Gastric hyperplastic polyps usually appear as multiple small sessile polyps.
2.
Gastric adenomatous polyps have a risk of malignant degeneration which is dependent on their size.
3.
Since the prediction of histologic type is often difficult on imaging study, the possibility of associated cancer foci or early gastric cancer should be suspected for any polypoid lesion with larger than 1–2 cm in size.
4.
For evaluation of gastric cancer on MDCT, it is crucial to obtain the adequate gastric distension in order to detect the primary lesion or avoid the false lesion.
5.
Both EGC and AGC have various morphologic types and imaging findings.
6.
In order to predict the accurate T stage on CT, it is important to understand the multilayered pattern of the normal gastric wall on CT.
7.
The mucinous adenocarcinomas of the stomach appear as fungating or diffusely infiltrative tumor with thickened low-attenuating middle or outer layer and sometimes have punctate or miliary calcifications.
8.
Gastric neuroendocrine tumors can give early hepatic metastases and regional lymph node metastases even in small size.
6.4 Illustrations: Mucosal Tumor of the Stomach
6.4.1 Three Hyperplastic Polyps in the Gastric Antrum
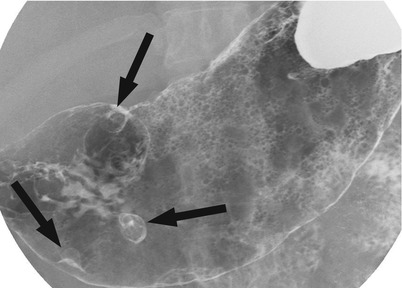
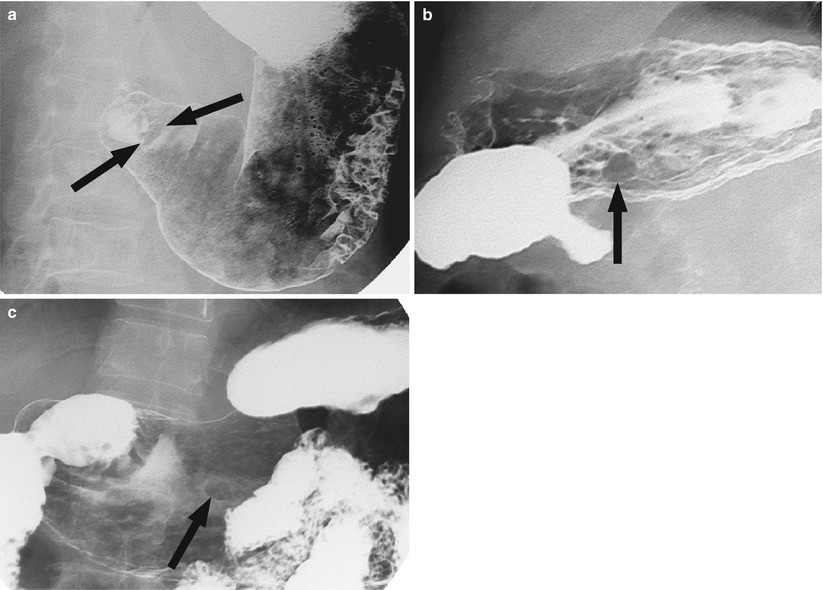
Fig. 6.1
Three hyperplastic polyps in the gastric antrum. There are three sessile polyps (arrows) in the antrum
6.4.2 Multiple Fundic Gland Polyps
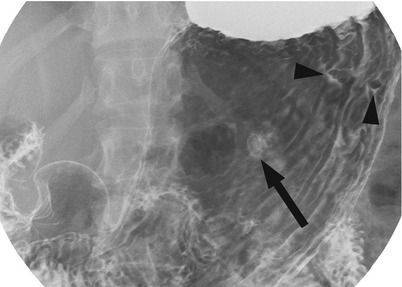
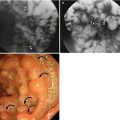
Fig. 6.2
Multiple fundic gland polyps. There are three small sessile polyps in the gastric body. One polyp in the anterior wall demonstrates the etched-in-white appearance (arrow) and two polyps in the posterior wall show filling defect (arrowheads)
6.4.3 Adenomatous Polyp