Fig. 1
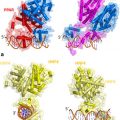
MAGE-A11 amplification of human AR transcriptional activity by linking AR dimers. AR dimerization is mediated by the DNA binding domain and the androgen-dependent AR N/C interaction (Wong et al. 1993) . MAGE-A11 binds the AR NH2-terminal 23FXXLFXXVXXV33 motif sequence 23FQNLFQSVREV33 (Bai et al. 2005; Liu et al. 2011) . The ability of MAGE-A11 to rescue the transcriptional activity of complementary inactive AR mutants that lack DNA binding or transcriptional activation domains suggests that MAGE-A11 dimers link AR dimers (Bai and Wilson 2008, Minges et al. 2013) . The ability of MAGE-A11 to increase AR transcriptional activity by linking AR dimers supports the dual function of the AR NH2-terminal FXXLF motif region in the androgen-dependent N/C interaction with activation function 2 in the ligand binding domain and an interaction site for MAGE-A11. This research was originally published in the Journal of Biological Chemistry. Minges et al. 2013 © the American Society for Biochemistry and Molecular Biology
MAGE-A11 also increases AR transcriptional activity by recruiting p300, a potent acetyltransferase that acetylates histones and transcription factors (Fig. 2) (Askew et al. 2010) . MAGE-A11 also helps to recruit p160 nuclear receptor coactivators through a direct interaction and by competing with the AR N/C interaction (Askew et al. 2009). The carboxyl-terminal region of MAGE-A11 contains a MAGE homology domain highly conserved across the MAGE gene family. The MAGE homology domain in MAGE-A11 interacts with the AR FXXLF motif region, is modulated by ubiquitination, and contains an F-box-like sequence phosphorylated by the cell cycle checkpoint kinase Chk1 (Bai and Wilson 2008). MAGE-A11 expression also appears to be cell cycle regulated (Bai and Wilson 2008; Askew et al. 2010). The predicted unstructured MAGE-A11 NH2-terminal region and highly structured carboxyl-terminal region are consistent with the crystal structure of MAGE-G1, another MAGE gene family member. The MAGE-G1 carboxyl-terminal MAGE homology domain contains multiple interacting α-helices linked to an unstructured NH2-terminal region not detected in the structure analysis (Doyle et al. 2010) . (Fig. 2)
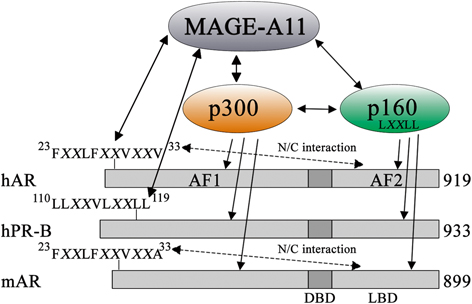

Fig. 2
MAGE-A11 increases transcriptional activation of human AR and human PR-B. MAGE-A11 interacts with the human AR (hAR) NH2-terminal FXXLF motif region 23FXXLFXXVXXV33 and human PR-B (hPR-B) NH2-terminal 110LLXXVLXXLL119 region, and increases transcriptional activity by recruiting p300 and p160 nuclear receptor coactivators. MAGE-A11 binding to the AR FXXLF motif region competes with AR FXXLF motif binding to activation function 2 (AF2) in the ligand binding domain (LBD) that mediates the androgen-dependent AR N/C interaction. Mouse AR (mAR) and AR from other less evolved mammals have 23FXXLFXXVXXA33 NH2-terminal sequence with Ala-33 instead of Val-33 in primate AR, which undergoes an N/C interaction but does not interact with MAGE-A11. The human AR-V33A mutation inhibits interaction with MAGE-A11. Coevolution of MAGE-A11 and AR is suggested by the primate-specific expression of MAGE-A11 and the primate-specific AR NH2-terminal sequence required to interact with MAGE-A11. This research was originally published in the Journal of Biological Chemistry. Liu et al. 2011 © the American Society for Biochemistry and Molecular Biology
5 Regulation of Human Progesterone Receptor-B by MAGE-A11
Cancer-testis antigens and the MAGE-A family are sometimes described as expressed exclusively in cancer (Sang et al. 2011) . However, proteins expressed in cancer can have functions in normal cells during development or at times of rapid cell growth or differentiation. The full developmental spectrum of MAGE-A11 expression in humans remains to be examined rigorously. The MAGE-A11 gene promoter contains a CpG island subject to extensive DNA methylation that limits expression in normal tissues. However, there are examples of MAGE-A11 expression in normal tissues of the human reproductive tract.
MAGE-A11 is expressed in normal human endometrium during the menstrual cycle. MAGE-A11 mRNA increases 20-50-fold in mid-secretory endometrial biopsies obtained from normal cycling women (Bai et al. 2008) . MAGE-A11 immunostaining increases in mid-secretory endometrial epithelial cells. Highest levels of MAGE-A11 mRNA and protein are in the mid-secretory window of implantation. MAGE-A11 expression in human endometrium increases after the surge in luteinizing hormone (LH) that follows ovulation. Up-regulation of MAGE-A11 mRNA by cyclic AMP in endometrial and prostate cancer cell lines (Bai et al. 2008; Karpf et al. 2009) suggests that the LH-induced increase in cyclic AMP increases MAGE-A11 expression in the mid-secretory endometrium and may be required to establish receptivity for embryo implantation in humans. The hormone-dependent cycling of human endometrium does not occur in less evolved mammals. The hormone-regulated expression of MAGE-A11 suggests that MAGE-A11 evolved in primates for successful reproduction.
Progesterone receptor (NR3C3)-A (PR-A) and PR-B are isoforms transcribed from the same gene using different promoters (Richer et al. 2002) . PR-B is identical to PR-A except for 164 extra NH2-terminus amino acids. PR-A is the predominant isoform in mouse uterus, whereas the more transcriptionally active PR-B is predominant in human endometrium (Igarashi et al. 2005) . MAGE-A11 increases the transcriptional activity of PR-B in an isotype-specific manner by interacting with a unique PR-B NH2-terminal 110LLXXVLXXLL119 motif (Fig. 2) (Su et al. 2012) . The transcriptional enhancing effects of MAGE-A11 for PR-B were not seen with PR-A or the PR-A/B heterodimer. MAGE-A11 interaction with human PR-B is regulated by phosphorylation and ubiquitination, functions synergistically with p300, and amplifies the coregulator effects of p160 nuclear receptor coactivators. Interactions between MAGE-A11 and PR-B may explain the greater transcriptional activity of PR-B relative to PR-A. A ligand-dependent N/C interaction reported for PR-B (Tetel et al. 1999) may be modulated by MAGE-A11.
The coregulator function of MAGE-A11 therefore includes human AR and human PR-B. MAGE-A11 may function primarily as a PR-B coregulator in human endometrium, although effects on AR in uterus have not been excluded. The hormone-regulated cyclic function of human endometrium and evolution of MAGE-A11 is consistent with its function as an amplifying signaling molecule for steroid hormone receptors.
6 MAGE-A11 Interacting Partners in the Retinoblastoma Family
Cancer cells interfere with the retinoblastoma (Rb) family of proteins to achieve uncontrolled growth. The Rb family consists of the Rb tumor suppressor, p107 and p130. The major function of the Rb-related proteins is to regulate cell growth by inhibiting the activity of E2F transcription factors that drive the cell cycle. Inactivation of Rb family members in cancer occurs through several mechanisms. Rb gene mutations are common in late stage cancer and block the ability of Rb to inhibit E2F transcription factors resulting in cell cycle progression (Chellappan et al. 1991) . DNA tumor viruses, such as adenovirus early protein E1A, inactivate Rb family members to release transcriptionally active E2Fs and promote transition through the G1/S phase of the cell cycle (Fattaey et al. 1993) . MAGE-A11 interacts selectively with p107 of the Rb family, stabilizes p107 by inhibition of ubiquitination, and increases the transcriptional activity of E2F1 (Su et al. 2013) . MAGE-A11 interacts with hypophosphorylated E2F1 and does not interact with hyperphosphorylated E2F1, suggesting it is directly involved in E2F1 activation.
The ability of MAGE-A11 to increase E2F1 transcriptional activity resembles the activity of adenovirus early protein E1A, a DNA tumor virus protein that promotes cell transformation by disrupting Rb-related protein interactions with E2F transcription factors (Liu and Marmorstein 2007) . The specificity of MAGE-A11 interaction with p107, and its ability to increase E2F transcription factor activity, suggest that MAGE-A11 promotes cell cycle progression independent of its function as a steroid hormone receptor coregulator. MAGE-A11 may also provide an important link between steroid receptor transcriptional activity and cell cycle regulation.
Part III
AR and MAGE-A11 in Prostate Cancer
7 AR Activation in Castration-Recurrent Prostate Cancer
Castration-recurrent prostate cancer growth during androgen deprivation therapy is a major clinical challenge. AR continues to drive the growth of castration-recurrent prostate cancer based on the expression of androgen-regulated genes, and inhibition of prostate cancer cell growth by silencing AR (Gregory et al. 1998; Zegarra-Moro et al. 2002; Ponguta et al. 2008) . AR coregulators such as p160 nuclear receptor coactivators are often overexpressed in castration-recurrent prostate cancer (Gregory et al. 2001a). However, it remains controversial whether AR splice variants exist and are at sufficiently high levels to impact castration-recurrent prostate cancer (Dehm et al. 2008; Sun et al. 2010) . AR splice variants may be largely a phenomenon of prostate cancer cell lines that are subject to extensive gene rearrangements. The level of reported AR variant mRNA in tumor tissue is very low compared to full-length AR (Watson et al. 2010) and could represent incompletely processed RNAs detected using the sensitive technique of quantitative RT-PCR. AR is also highly susceptible to partial proteolysis during isolation, which generates forms that migrate as reported AR variants (Wilson and French 1979; Gregory et al. 2001b).
Continued AR signaling in castration-recurrent prostate cancer could represent to some extent ligand-independent AR activation. However, early studies raised the possibility of intratumoral DHT production from adrenal androgen precursors (Geller et al. 1978) . Improved techniques of radioimmunoassay and mass spectrometry demonstrated that testosterone and DHT are at sufficient concentrations to activate AR in castration-recurrent prostate cancer tissue in patients undergoing androgen deprivation therapy (Mohler et al. 2004; Titus et al. 2005; Mostaghel et al. 2007; Montgomery et al. 2008; Locke et al. 2008) . Adrenal androgen precursors of DHT synthesis circulate in the blood of patients undergoing androgen deprivation therapy by medical castration. Adrenal androgen supplementation increased prostate DHT in rodent models (Labrie et al. 1988) . Normal prostate and prostate cancer cells have enzymes that convert adrenal androgens to DHT (Mohler et al. 2011a, 2011b). Continued reliance on ligand-activated AR in recurrent prostate cancer growth suggests that adrenal androgens are important precursors for intratumoral DHT synthesis when circulating testosterone and DHT are low in patients undergoing androgen deprivation therapy. It has also been suggested that cholesterol may be a source of intratumoral androgen synthesis in castration-recurrent prostate cancer (Leon et al. 2010; Twiddy et al. 2011; Mostaghel et al. 2012) .
8 Increased Expression of MAGE-A11 in Castration-Recurrent Prostate Cancer
MAGE-A11 levels increase in prostate cancer during progression to castration-recurrent growth (Karpf et al. 2009) . Immunohistochemistry shows an increase in MAGE-A11 protein in the CWR22 human prostate cancer xenograft after castration of tumor-bearing mice and in clinical specimens of castration-recurrent prostate cancer. The increase in MAGE-A11 protein correlates with a 20–100 fold increase in MAGE-A11 mRNA in the CWR22 xenograft with time after castration. MAGE-A11 mRNA can increase up to 1000 fold in clinical specimens of castration-recurrent prostate cancer. An increase in MAGE-A11 mRNA was seen in ~ 30 % of castration-recurrent prostate cancer specimens analyzed, suggesting its overexpression represents one mechanism that contributes to castration-recurrent growth. MAGE-A11 is also overexpressed in epithelial ovarian cancers (James et al. 2013) .
The major cause of the increase in MAGE-A11 in prostate and ovarian cancer is hypomethylation of a CpG island at the transcription start site of the X-linked MAGE-A11 gene promoter (Karpf et al. 2009; James et al. 2013). Progressive hypomethylation of the promoter occurs with time after castration of mice with the CWR22 human prostate cancer xenograft. In a limited number of castration-recurrent prostate cancers analyzed, there was an inverse relationship between AR and MAGE-A11 mRNA levels. One castration-recurrent prostate cancer sample with undetectable AR mRNA determined using quantitative RT-PCR had 1000-fold higher levels of MAGE-A11 mRNA. This suggests a compensatory relationship between AR and MAGE-A11 contributes to prostate cancer growth.
MAGE-A11 mRNA also increases in response to cyclic AMP in human prostate cancer and endometrial cell lines (Bai et al. 2008; Karpf et al. 2009) . Cyclic AMP up-regulation of MAGE-A11 is another mechanism that increases MAGE-A11 in castration-recurrent prostate cancer because cyclic AMP levels increase in prostate cancer cells during androgen deprivation (Burchardt et al. 1999) . The increase in AR transcriptional activity in response to cyclic AMP (Merkle and Hoffmann 2011) may reflect an increase in MAGE-A11. MAGE-A11 also interacts with and stabilizes the ligand-free AR, which may account in some cases for the increase in AR in castration-recurrent prostate cancer. Higher levels of MAGE-A11 enhance androgen-stimulated AR transcriptional activity through interactions with p300 and p160 coactivators, and drive prostate cancer cell growth through its ability to sequester p107, increase E2F transcriptional activity that drives the cell cycle.
9 Proto-Oncogene Activity of MAGE-A11 in a Transcriptional Hub
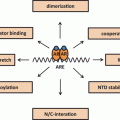
MAGE-A11 is a multifunctional protein expressed at low levels in normal human cells and at higher levels in cancer. Extensive DNA methylation of the promoter that limits MAGE-A11 expression in normal cells is reversed in cancer cells by DNA hypomethylation. The ability of MAGE-A11 to increase the transcriptional activity of AR and PR-B by interacting with unique NH2-terminal motifs also depends on its interaction with p300 and p160 nuclear receptor coactivators (Fig. 3). MAGE-A11 interacts with p107, a member of the Rb family that regulates cell growth by sequestering E2F transcription factors. MAGE-A11 stabilizes p107 in association with increased E2F1 transcriptional activity. Inhibition of prostate cancer cell growth by suppressing MAGE-A11 expression suggests a critical role in cell cycle regulation. The primate-specific expression of MAGE-A11 suggests it introduces a gain-in-function that enhances steroid hormone receptor transcriptional activity and provides a direct link to cell cycle progression. The multiple interacting partners suggest that MAGE-A11 functions in a transcriptional hub to increase steroid receptor activity and cell cycle progression in rapidly dividing normal cells and in the uncontrolled growth of cancer cells. Based on crystal structure of MAGE-G1 (Doyle et al. 2010) , MAGE-A11 may be a hub protein with ordered and disordered regions separated by flexible hinges similar to steroid receptors (Dunker et al. 2005) . (Fig. 3)
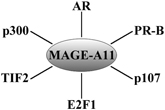

Fig. 3
MAGE-A11 in a transcriptional hub. MAGE-A11 interacts with multiple proteins involved in steroid hormone-regulated gene expression and cell cycle control. MAGE-A11 increases human AR and human PR-B transcriptional activity through direct interactions with NH2-terminal motifs and through interactions with the p300 acetyltransferase and p160 coactivator, transcriptional intermediary factor 2 (TIF2). MAGE-A11 increases cell cycle progression through an interaction with p107 of the retinoblastoma family, which increases E2F1 transcriptional activity. MAGE-A11 interacts selectively with hypophosphorylated E2F1. The multiple interactions of MAGE-A11 suggest its functions in a hub to increase gene transcription and cell cycle progression in primates
Acknowlegments
John T. Minges provided assistance with the figures and Frank S. French reviewed the manuscript. Research support was from National Institutes of Health Grant HD16910, National Cancer Institute Grant P01-CA77739, and United States Public Health Service grant HD067721 from NIH Eunice Kennedy Shriver NICHD Cooperative Agreement U54-HD35041 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
References
Askew EB, Gampe RT, Stanley TB, Faggart JL, Wilson EM (2007) Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J Biol Chem 282:25801–25816PubMedCentralPubMed
Askew EB, Bai S, Hnat AT, Minges JT, Wilson EM (2009) Melanoma antigen gene protein-A11 (MAGE-11) F-box links the androgen receptor NH2-terminal transactivation domain to p160 coactivators. J Biol Chem 284:34793–34808PubMedCentralPubMed
Askew EB, Bai S, Blackwelder AJ, Wilson EM (2010) Transcriptional synergy between melanoma antigen gene protein-A11 (MAGE-11) and p300 in androgen receptor signaling. J Biol Chem 285:21824–21836PubMedCentralPubMed
Askew EB, Minges JT, Hnat AT, Wilson EM (2012) Structural features discriminate androgen receptor N/C terminal and coactivator interactions. Mol Cell Endocrinol 348:403–410PubMedCentralPubMed
Bai S, Wilson EM (2008) Epidermal growth factor-dependent phosphorylation and ubiquitinylation of MAGE-11 regulates its interaction with the androgen receptor. Mol Cell Biol 28:1947–1963PubMedCentralPubMed
Bai S, He B, Wilson EM (2005) Melanoma antigen gene protein MAGE-11 regulates androgen receptor function by modulating the interdomain interaction. Mol Cell Biol 25:1238–1257PubMedCentralPubMed
Bai S, Grossman G, Yuan L, Lessey BA, French FS, Young SL, Wilson EM (2008) Hormone control and expression of androgen receptor coregulator MAGE-11 in human endometrium during the window of receptivity to embryo implantation. Mol Hum Reprod 14:107–116PubMedCentralPubMed
Bennett MJ, Schlunegger MP, Eisenberg D (1995) 3D domain swapping: a mechanism for oligomer assembly. Protein Sci 4:2455–2468PubMedCentralPubMed
Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R (1999) Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol 162:1800–1805PubMed
Chang CY, Walther PJ, McDonnell DP (2001) Glucocorticoids manifest androgenic activity in a cell line derived from a metastatic prostate cancer. Cancer Res 61:8712–8717PubMed
Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR (1991) The E2F transcription factor is a cellular target for the RB protein. Cell 65:1053–1061PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree