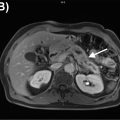
Primary bone and soft tissue malignancies
Primary bone and soft tissue malignancies are a heterogeneous group of relatively rare tumors, most of them deriving from mesenchymal tissue (sarcomas). The reported international annual incidence for sarcomas ranges from 1.8 to 5 cases per 100,000 individuals ( ). They can occur anywhere in the body but most commonly in the extremities (31.9%) followed by the trunk (21.8%) and abdomen (19.7%) ( ).
Morphologic imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) have been established as important tools in the evaluation of primary osseous tumors and soft tissue lesions. Aggressive features for bone lesions include ill-defined margins, extension into surrounding tissues, and osseous cortical disruption. Cortical expansion and remodeling are more commonly seen with less aggressive lesions ( ). Identification of these features prompts the need for biopsy, often leading to the initial histological diagnosis ( ). Malignant soft tissue lesions are widely variable in their appearance.
Hybrid PET modalities, such as PET/CT and more recently PET/MRI, continue to develop in their role for the evaluation of several malignancies including bone and soft tissue cancers, providing contributions to staging, identification of biopsy targets (favoring more metabolically active areas to minimize nondiagnostic sample or undersampling), prognosis, evaluation of treatment response, and assessment for recurrence.
The most widely employed PET radiotracer in routine clinical practice is 18 F-flurodeoxyglucose (FDG), which has revolutionized staging and evaluation of treatment response for many cancers. Other tracers that have been reported to be potentially helpful in these conditions include agents like 68 Ga- tetraazacyclododecanetetraacetic acid–DPhe1-Tyr3-octreotate (DOTATATE), prostate-specific membrane antigen (PSMA), and quinolone-based fibroblast activation protein inhibitor (FAPI) ( ; ; ). Regarding FDG, avidity ranges from minimal to intense both in malignant and benign lesions and can also be present in inflammatory conditions ( ; ). There is overlap uptake between low-grade sarcomas and benign lesions. Therefore, interpretation of hybrid imaging requires that metabolic findings are assessed in conjunction with morphologic findings, along with the specific clinical data Table 8.1 .
| Intermediate-High FDG avidity | Low FDG avidity | |
|---|---|---|
| Malignant | OSA, Ewing sarcoma, chondrosarcoma (high grade or dedifferentiated) | MLS , chondrosarcoma (low or intermediate grade) |
| Benign | NOF, GCTB, FD, Paget disease of bone (active phase), enchondroma, liposclerosing myxofibrous tumor, sarcoidosis, EG, chondroblastoma | Hemangioma, osteochondroma, OO, enchondroma, bone cyst, intramuscular myxoma |
Hematopoietic bone marrow, which is more abundant in the spine and pelvis in adults, has greater cellularity, higher metabolism, and therefore greater FDG avidity, than fatty marrow found elsewhere in the skeleton ( ).
When using somatostatin receptor PET tracers (e.g., DOTATATE), non-neuroendocrine lesions such as phosphaturic mesenchymal tumors, hemangiomas, epithelioid hemangioendotheliomas, and meningiomas will also show uptake.
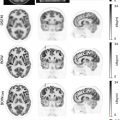
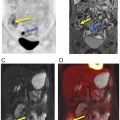
FAPI is an interesting radiopharmaceutical which might overcome several limitations faced by FDG, especially the FDG uptake in bone marrow and the low FDG uptake in low-grade sarcomas. In initial studies, FAPI SUVmax has been among the highest (>12) in sarcomas ( ) ( Fig. 8.1 ). Background uptake has been negligible (<1.6), including most normal muscles, bone marrow, and blood. Tumor to background ratio has been reported >6 in high intensity uptake malignancies, such as sarcomas ( ). However, large muscles, degenerative bone conditions, and muscle scarring might demonstrate higher FAPI uptake, with SUVmax up to 7.7, which, however, is still well below the SUVmax reported so far in the cases of sarcomas ( ; ). The synergistic information from morphologic and functional features from MRI and metabolic data from FAPI-PET, might make FAPI-PET/MRI an ideal technology to study sarcomas, including the low-grade grade ones, to distinguish sarcomas from benign pathologies, to delineate local extension, and improve metastases detection. A single FAPI-PET/MRI examination could potentially provide all the necessary fine anatomic details needed for surgical and radiation therapy planning, along with the benefits of an accurate whole body staging ( Fig. 8.2 ) ( ).


The functional multiparametric capabilities of MRI provide additional information to complement its excellent contrast and spatial resolution. For example, diffusion-weighted imaging (DWI) may help distinguish necrosis and fibrosis from viable tumor. Apparent diffusion coefficients (ADC) from DWI have shown promise in distinguishing between high-proliferation and low-proliferation soft tissue sarcomas (STS) ( ). Studies on dynamic contrast-enhanced (DCE) MRI have reported a potential role in the early prediction of histologic response in STS ( ), and in prognostication for osteosarcomas (OSA) ( ). Early work on magnetic resonance spectroscopy has revealed that choline peaks were higher in malignant compared to benign musculoskeletal lesions ( ). High choline peaks can also be found in giant cell tumors of bone ( ) which is considered a benign tumor but may be locally aggressive or metastasize. PET/MRI, combining these capabilities with metabolic imaging, has the potential to improve the assessment of treatment response and detection of recurrence. Besides these benefits, PET/MRI is associated with decreased radiation dose (30%–75%, depending on protocol and equipment) when compared to PET/CT ( ; ).
The literature on PET/MRI in primary osseous malignancy and soft tissue sarcoma is limited and mostly related to FDG-PET/CT. Significant improvements in MRI attenuation correction methods have allowed for robust evaluation of osseous structures and the surrounding soft tissues ( ; ; ). Protocols and indications are still in development, and technologists with expertise in PET imaging and musculoskeletal MRI will be critical components for success.
Staging
For primary bone tumors and STS, local staging is typically carried out using MRI, which allows for excellent delineation of tumor margins and can identify involvement of important adjacent structures such as neurovascular elements, fascial planes, and joints ( ). This information is vital for surgical and radiation treatment planning. Since tissue heterogeneity is common in high grade sarcomas, metabolically active regions within the primary tumor can be identified by FDG-PET and targeted with percutaneous sampling to ensure a diagnostic biopsy and avoid understaging ( Fig. 8.3 ).

Accurate local staging is crucial since incomplete resection may lead to local recurrence and decreased overall survival ( ). Nodal staging capabilities of CT and MRI can be improved when combined with PET ( ). In relation to metastatic staging, CT carries an advantage over MRI in the detection of pulmonary metastases (especially when smaller than 10 mm) ( ), alternatively, MRI may have greater capabilities in the detection and characterization of liver, brain and, bone lesions ( ; ; ; ; ; ; ; ; ).
Few studies are available evaluating the utility of PET/MRI in staging STS ( ; ; ). When comparing the performance between FDG-PET/MRI and FDG-PET/CT for the evaluation of malignant bone lesions and STS, while lesion conspicuity was comparable between both hybrid imaging modalities ( ; ), T1-weighted MRI images were superior to CT for the anatomic delineation of osseous lesions which may be important in the early infiltration of bone marrow and in lesions with low radiotracer avidity ( ). Studies of pediatric rhabdomyosarcoma have shown that FDG-PET/CT was superior to conventional imaging with MRI and CT, resulting in changes to lymph node staging and osseous involvement ( ; ). There are no well-defined guidelines on the use of hybrid PET imaging modalities for bone and STS. However, some authors acknowledge a role as a problem-solving tool when conventional anatomic imaging is equivocal. It should be noted that for low-grade sarcomas, which may show low FDG avidity, the potential value in staging is like to be of limited benefit with specific radiopharmaceutical. However, other agents, like FAPI, seem promising.
Monitoring treatment response and recurrence detection
Given the excellent soft tissue contrast resolution, MRI is the modality of choice to evaluate for treatment-related changes and local recurrence and is favored over CT ( ). When neoadjuvant RT is used, imaging should be delayed by approximately 6–8 weeks to allow for a more accurate interpretation of the imaging findings ( ). Changes in size might not reflect histopathological response, therefore additional characteristics such as changes in metabolism as evaluated with hybrid PET modalities, differences in MRI signal/enhancement and ADC can be used to assess treatment effects ( ; ). These need to be interpreted in the context of the specific treatment and tumor histology to reach meaningful conclusions ( ; ; ; ). For example, adapted Choi criteria propose that the absence of new lesions and decrease in enhancement ≥15% suggests partial response, and alternatively, a ≥15% increase in enhancement suggests progressive disease. This is considered to be more accurate than RECIST criteria for assessing high-grade STS ( ). When using FDG-PET/CT, a reduction in ≥35% of standard uptake value (SUV) peak at early follow up has been proposed as a sensitive predictor of histologic response ( ).
In a series of 26 patients with OSA of the extremities, FDG-PET/MRI revealed that changes in metabolic activity after neoadjuvant chemotherapy could be used as a surrogate of treatment-induced tumoral necrosis ( ). Another series of 45 patients with a diagnosis of STS who underwent FDG-PET/MRI showed superior assessment of treatment response compared to stand-alone MRI ( ). PET/MRI might also be useful when evaluating complex anatomic regions, such as the brachial plexus with reported excellent delineation of residual malignancy in this location ( ).
Recurrence in STS occurs in 17%–39% of cases ( ). Diagnostic challenges include distinguishing scar tissue and recurrent tumor, decreasing delayed detection of recurrence, and minimizing unnecessary medical intervention. A study by Erfanian et al. that evaluated 41 patients with a diagnosis of STS, described a role for the evaluation of recurrence, citing improved sensitivity, specificity, and confidence levels when using FDG-PET/MRI compared to MRI alone ( ). The sensitivity and specificity of MRI alone for detection of STS recurrence is 92% and 98%, respectively, however, for subcutaneous primary tumors such as myxofibrosarcoma and after radiation therapy, sensitivity and specificity decrease to 83% and 86%, respectively ( ). The combination of metabolic information from PET with the morphofunctional data of MRI provides a promising tool for the accurate detection of recurrent disease.
Prognosis
In adults with STS, overall survival rates at 5 years range from 60% to 80% ( ). For bone and joint cancer, 5-year relative survival is 67%. Overall prognostic factors include patient age, tumor grade, tumor depth and size, histologic subtype, surgical margins at resection, and recurrent versus primary disease status ( ). Currently, there is paucity of data on the role of PET/MRI in the prognosis of primary bone and soft tissue malignancies. On the other hand, several PET/CT studies support prognostic capabilities in STS. With the use of semiquantitative measurements such as SUVmax, hybrid PET modalities have a role in the prediction of biological activity and tumor grade in bone sarcoma and soft tissue sarcoma. High SUVmax and total lesion glycolysis measured on FDG-PET/CT before and after initial chemotherapy for OSA were associated with worse progression-free survival and poor overall survival ( ). Studies have also shown similar capabilities in chest wall, limb, and girdle sarcomas ( ; ). A study evaluating the prognostic value of FDG-PET for resectable STS reported that 5-year recurrence-free survival rates in patients with SUV mean of <1.59, 1.59–3.59, and ≥3.6 were 66%, 24%, and 11%, respectively, concluding that semiquantitative analysis is a useful prognostic parameter ( ). A meta-analysis reported that high SUVmax was associated with shorter overall survival in bone sarcoma and STS ( ).
Primary bone and soft tissue lesions
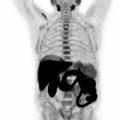
OSA is the most common primary skeletal malignancy, more frequently seen in patients between 10 and 20 years of age with a small increase in incidence after 60 years of age. Most OSAs arise from the metaphyses of long bones. Conventional OSA accounts for 75% of all OSA and is a high-grade tumor. Lesions typically show ill-defined margins, aggressive periosteal reaction (60% of cases), and variable degrees of extraosseous extension (75% of cases) ( ). MRI is especially useful in the evaluation of extraosseous extension and detection of potential additional foci in the same or adjacent bones (skip lesions) ( Fig. 8.4 ).

In the case of neoadjuvant chemotherapy for OSA, treatment-induced necrosis greater than 90% is considered a good response ( ; ). Neoadjuvant treatment response has been classically assessed with histopathologic analysis; however, FDG hybrid PET imaging may have a role in this setting with a study reporting SUVmax >5 in histologic nonresponders using 18 F-FDG PET/CT ( ).
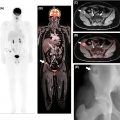
Ewing sarcoma, part of the small round cell sarcomas, has a predilection for flat bones and the diaphysis of long bones, despite being capable of originating from any part of the skeleton. Approximately 80% of these malignancies occur in patients younger than 20 years of age. Lesions are typically large and destructive ( ) ( Fig. 8.5 ).

As with many other lesions, MRI has a crucial role in evaluating extraosseous extension and lesion enhancement (before and after chemotherapy) ( ). Ewing sarcoma metastases typically occur in the lungs and other bones. As with OSA, MRI and FDG-PET/CT play a role in assessing treatment response and in detecting recurrent disease.
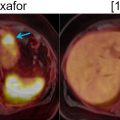
Giant cell tumor of bone (GCTB) is a rare, locally aggressive tumor that typically occurs in the bones of skeletally mature young adults, in their second to fourth decades. Lesions typically originate from the metaphysis but extend to the epiphysis, may expand the bone, elevate the periosteum, and demonstrate well-defined medullary margins. GCTBs show a lytic appearance on CT and exhibit intermediate-low signal on T1 and T2-weighted MRI images ( ). Treatment often includes curettage and reconstruction. Systemic treatment options like Denosumab (RANK ligand inhibitor) can reduce tumor size, slow down progression and increase intralesional bone density and mineralization. As in many other tumors, a decrease in FDG metabolism after treatment with Denosumab, often precedes morphological changes, demonstrating a role of PET in assessing treatment response ( ; ).
Chondrosarcoma is more commonly seen in older adults (50–80 years of age) and is a malignant cartilaginous matrix producing tumor. There is increased risk for developing chondrosarcoma in patients with Maffucci syndrome and Ollier’s disease ( ) PET/CT may be helpful in cases of high-grade or differentiated chondrosarcoma ( ). Some authors have proposed a complementary role of FDG-PET/CT to conventional morphologic imaging in differentiating chondromas from chondrosarcomas in long bones, with SUVmax >2.0 favoring chondrosarcomas ( ). Similarly, a patient series reported SUVmax of 0.8–1.3 for benign osseocartilaginous lesions, SUVmax of 3.3–6.9 for grade II chondrosarcoma, and SUVmax of 11.4 in a dedifferentiated chondrosarcoma ( ).
Chordoma is a rare tumor that arises from the axial skeleton (more common in the sacrum and clivus), reflecting its origin from notochordal cells. Most are diagnosed between 40 and 80 years of age. Lesions typically appear destructive and show prominent extraosseous components ( ). On MRI, chordoma appears largely hyperintense on T2-weighted images and presents heterogeneous (often mild) contrast enhancement. Chordomas typically present moderate heterogeneous uptake on FDG-PET/CT ( ). Chordoma may also be evaluated with 68 Ga DOTATATE ( ).
Approximately 50% of STS occur in the extremities and 15% in the trunk. These include nearly 100 different histologic types ( ). In the trunk and extremities of adults, the most common entities are undifferentiated pleomorphic sarcoma (UPS), leiomyosarcoma, liposarcoma and synovial sarcoma. The imaging appearance of these tumors is widely variable and to some extent dependent on their subtype. Features such as peritumoral edema and necrosis are often seen with MRI in high-grade sarcomas ( ). Because of their low cellularity, lesions such as myxoid liposarcoma and intramuscular myxoma may have overlapping appearance on FDG-PET/CT, both presenting low metabolism ( ). MRI may distinguish between these entities if macroscopic fat can be identified or if enhancing nodular components are present. Therefore, in several instances, tissue diagnosis is necessary even after comprehensive imaging. In STS, nodal metastases are relatively uncommon but within this group are more often present in rhabdomyosarcoma and synovial sarcoma.
Metastatic disease to the skeleton
18 F FDG PET/MRI
The role of FDG-PET/CT has long been established for the staging and restaging of several malignancies, including breast cancer, lymphoma, small/non-small-cell lung cancer, head and neck squamous cell cancer, soft tissue/bone sarcomas, melanoma, and several enteric malignancies ( , ; ; ; ; ; ). Relatively few studies have focused on the added value of PET/MRI over PET/CT for evaluation of skeletal metastatic disease. In addition to improved detection of marrow replacing lesions, a potential benefit of the MRI component of PET/MRI is the ability to distinguish benign processes such as benign lesions or fractures from metastases ( ; ; ; ) ( Fig. 8.6 ).

Several studies compared FDG-PET/MRI and FDG-PET/CT for the evaluation of osseous metastases in breast cancer, showing a similar, to slightly increased, sensitivity of PET/MRI versus PET/CT ( ; ; ) ( Fig. 8.7 ).

They show an increased sensitivity in detecting bony lesions with a per lesion sensitivity in the range 92%–99% for PET/MRI and 69%–87% for PET/CT. The increased sensitivity for individual osseous lesions may be related to the higher contrast resolution of the MRI portion of the study in comparison with CT ( ). In one study, in addition to detecting more bone lesions than same-day PET/CT (141 compared to 90 lesions), PET/MRI was also positive for skeletal metastatic disease in 12% of patients with negative PET/CT ( ). Detection of these lesions, which would have otherwise been missed, may potentially influence clinical management.
Non 18 F-FDG tracers
18 F-Sodium fluoride: The clinical use of 18F-Sodium fluoride (NaF) PET/CT is recommended by the NCCN guidelines for evaluation of osseous lesions in metastatic or locally advanced breast cancer ( ). Of note, false positive uptake can be seen in benign conditions associated with increased osteoblastic activity such as degenerative and inflammatory changes, osteonecrosis, fractures, and benign osseous tumors ( ). However, to the best of our knowledge there are no published data on the use of NaF-PET/MRI in humans at this date.
18 F-Fluciclovine: 18F-Fluorocyclobutane-1-carboxylic acid (18F-FACBC or 18F-fluciclovine) is a radiolabeled synthetic amino acid analog that localizes to areas of increased amino acid transport. 18F-fluciclovine has shown promise for evaluation of prostate adenocarcinoma ( ) ( Fig. 8.8 ).

18F-fluciclovine PET/MRI has been shown to identify lymph node or soft tissue disease ( ). Because patients with known osseous metastasis were excluded from early trials of 18 F-fluciclovine PET in prostate cancer, there is less evidence for the value of this radiopharmaceutical for skeletal metastases ( ; ; ). However, available data shows superiority of 18 F-fluciclovine compared to 99m Tc bone scintigraphy, allowing for detection of early marrow replacing lesions ( ). 18 F-fluciclovine PET/CT tends to be less sensitive in small densely sclerotic lesions ( ).
A recent study explored the performance of 18 F-fluciclovine PET/MRI for the detection of osseous metastasis in castration-resistant prostate cancer. The results suggested a high sensitivity of 18 F-fluciclovine PET/MRI, and MRI alone for the detection of osseous metastases. This study also confirmed that 18 F-fluciclovine PET alone underperformed in the detection of late sclerotic lesions ( ).
68 Ga and 18 F -PSMA: PSMA radiopharmaceuticals labeled with 68Ga and 18F target cell surface receptors and are useful in investigating prostate cancer ( ). A recent study showed equivalent accuracy of PSMA-PET/MRI and PET/CT for the detection of bone metastases in patients with prostate cancer ( ) ( Fig. 8.9 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree