Ischemic heart disease is the leading cause of death in the United States and Europe. Advances in therapy have resulted in improved survival of patients suffering from acute myocardial infarction (MI). However, this improvement has led to an increase in the number of patients surviving with chronic coronary artery disease (CAD). Many of these patients require revascularization. Some may develop complications such as thrombus formation or ventricular aneurysm. Other patients present with chronic exercise-induced chest pain (angina) and may require revascularization, depending on their residual viable myocardium and coronary artery anatomy. Ischemic heart disease is a leading cause of heart failure, and differentiation from other cardiomyopathies that may cause heart failure is paramount to guide appropriate therapy.
Since 2000, substantial progress has been made in magnetic resonance imaging (MRI) hardware and software development such that cardiovascular MRI can now be considered a first-line modality in the evaluation of many facets of ischemic heart disease. For example, advances in coil design coupled with parallel acquisition techniques have resulted in significant shortening of examination time. A complete cardiac examination can now be performed in as little as 30 to 45 minutes. New pulse sequences provide improved visualization of irreversibly scarred myocardium in vivo with a precision that is unparalleled by any other modality. This chapter reviews the information MRI can provide that is useful in the evaluation of ischemic heart disease and its complications.
Ischemic Cascade
When considering the utility of cardiac magnetic resonance (CMR) imaging for the evaluation of ischemic heart disease, it may be useful first to consider the effects of ischemia on the myocardium at a tissue level because many of these events have MRI correlates. Myocardial ischemia reflects an imbalance between myocardial oxygen supply and demand, most often (but not exclusively) as a result of CAD. The initiating event in this ischemic cascade is the reduction of perfusion to less than the level needed to support myocardial metabolism. This process causes metabolic disorders at the tissue level, which then result in diastolic dysfunction, followed shortly by systolic dysfunction. If the imbalance persists, electrocardiogram (ECG) abnormalities may ensue, and symptomatic chest pain may develop.If the ischemic deficit is of sufficient severity and duration, MI will result. This deficit occurs first in the subendocardium, but with prolonged ischemia, it may extend to involve the full thickness of the myocardium in a given segment. Indeed, if the ischemic injury persists for a period of time without restoration of normal epicardial coronary flow, not only myocytes supplied by the respective artery die, but also endothelial cells at the precapillary and capillary level in the region of infarction may undergo necrosis. In the acute phase, the infarcted myocardium is usually thickened and edematous, whereas chronic MIs typically show thinning of the affected segments, with diminished function.
Restoration of flow may not immediately result in restoration of function even in the absence of MI because the metabolic deficit may take some time to recover. Myocardial stunning describes reversible myocardial dysfunction resulting from an acute ischemic insult that resolves before myocardial necrosis in the affected tissue. In addition, a slowly developing, chronic reduction in myocardial blood flow has been postulated to result in myocardial “hibernation,” a state of chronic myocardial dysfunction often accompanied by thinning of the affected segments. This state is believed to result from a downregulation of myocyte metabolism and ultrastructure to match its chronically impaired blood supply. This condition is also thought to be a reversible form of myocardial dysfunction.
The foregoing physiologic and anatomic abnormalities that are caused by ischemic injury can be well characterized using a variety of CMR techniques. For example, perfusion MRI allows detection of impaired perfusion occurring as a result of a significant coronary artery stenosis. Areas of segmental wall motion abnormality representing systolic and diastolic dysfunction can be clearly visualized using cine MRI. Global and regional myocardial function can be quantified accurately. MI can be visualized in both the acute and chronic phases by using the delayed enhancement MRI (DE-MRI) technique, which is widely recognized as the gold standard imaging test for the detection of MI and for the determination of myocardial viability ( Table 28-1 ).
| Physiologic Abnormalities | CMR Imaging Correlates |
|---|---|
| Decreased perfusion | Focal deficit on perfusion imaging |
| Metabolic derangements | Spectroscopic abnormalities (research) |
| Wall motion abnormalities | Cine imaging abnormalities (regional or global) |
| Myocardial infarction | Delayed enhancement on DE-MRI |
| Microvascular injury | No-reflow zone on DE-MRI |
In the case of myocardial thinning and dysfunction, cases caused by potentially reversible hibernation can be clearly distinguished from those resulting from irreversible scar. These are important distinctions that in many cases can be made only with MRI.
Given the multiple techniques available, and the variety of clinical situations to which they can be applied, the difficulties in standardizing cardiac MRI protocols for the evaluation of ischemic heart disease are not surprising. However, the Society of Cardiovascular Magnetic Resonance developed suggestions for standard protocols, which are reviewed in the following sections. As an important modification of the standard examination, stress perfusion MRI is also discussed in some detail.
Standard Cardiovascular Magnetic Resonance Examination
Figure 28-1 illustrates many of the components, along with a timeline, of a typical multitechnique CMR protocol for cardiac imaging. Sequences are added or excluded depending on the indication, patient considerations (e.g., the ability to breath hold), and even findings during the examination itself. Generally, all patients undergo cine imaging for the assessment of morphology, ventricular volumes, and contractile function. These images are typically acquired in 6- to 8-mm short-axis sections at contiguous 1-cm intervals from the mitral valve plane through the apex, as well as in the standard long-axis views (i.e., paraseptal long-axis, four-chamber, three-chamber views). DE-MRI after gadolinium administration is routinely performed with similar section thickness in locations spatially matched to the cine images. This technique allows the diagnosis and sizing of MI, assessment of viability, and other tissue characterization such as identification of thrombus and nonischemic scarring. Irregular heart rhythm or an inability to cooperate with breath holding may necessitate the use of single shot sequence variants to obtain diagnostic-quality images.
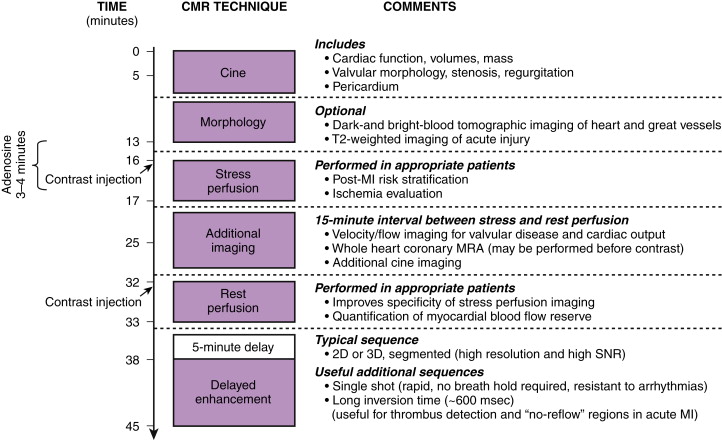
Optional elements include stress perfusion imaging to evaluate ischemia and velocity-encoded imaging for the assessment of hemodynamics and valvular function. Additionally, T2-weighted imaging has shown promise in assessing acute, inflammatory processes such as acute MI or myocarditis, and it may prove useful in distinguishing chronic lesions from those of recent onset. At experienced centers, coronary magnetic resonance angiography (MRA) may be performed to exclude left main or three-vessel CAD in selected patients or to evaluate coronary anomalies.
Findings in Ischemic Heart Disease
Acute Ischemic Injury
Cine Imaging
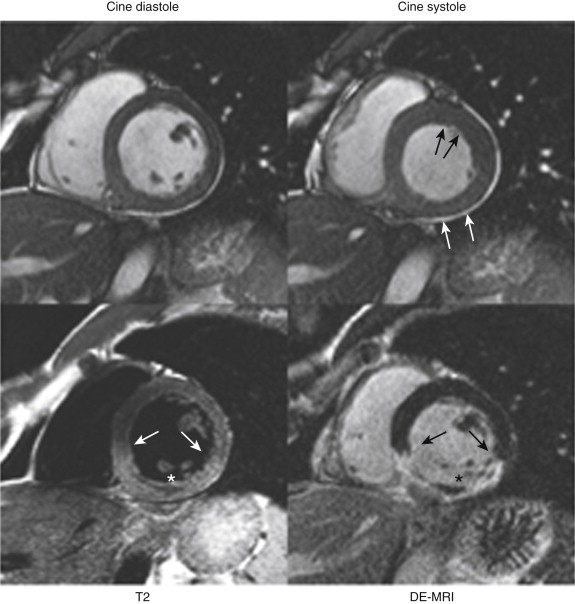
Acute ischemic injury of the myocardium usually results in myocardial dysfunction, with reduction of systolic wall thickening (not thickness) evident on cine images. This change may occur even in the absence of infarction, as expected from the discussion of the ischemic cascade. Acute infarctions may result in transient swelling of the affected segment, with increased signal often apparent on steady-state free precession (SSFP) cine imaging that reflects the presence of edema ( Fig. 28-2 ).
Delayed Enhancement Magnetic Resonance Imaging
Acute ischemic injury that does not cause infarction does not result in hyperenhancement. Acute infarctions are recognized as focal areas of subendocardial hyperenhancement on DE-MRI, and they may extend in a transmural fashion for a variable distance, depending on the duration and severity of the ischemia. More severe and long-standing ischemic episodes usually result in a greater transmural extent of infarction because necrosis proceeds in a “wavefront” fashion from the subendocardium toward the epicardium. If the injury is sufficiently severe, it may result in the development of a no-reflow zone, visible on DE-MRI as a dark, nonenhancing core at the center of an extensive infarction. These regions (discussed in more detail later in the chapter) are the DE-MRI correlates of microvascular occlusion from an ischemic injury that is sufficiently severe to cause endothelial damage in the core of the infarct (see the earlier section on the ischemic cascade).
T2 Imaging
In the setting of acute myocardial injury, increased signal may be seen in areas of infarction, thus reflecting the presence of edema (see Fig. 28-2 ). Investigators have proposed that the area at risk, but not infarcted, may be discerned by comparison of the edematous areas on T2-weighted images with the DE-MRI images, but this is controversial.
Chronic Ischemic Injury
Cine Imaging
Chronic ischemic injury may result in impaired function, with regional or global hypokinesia, or even akinesia or dyskinesia. Wall thinning may also occur in the affected segments and may result from earlier infarction; however, it can also be seen with hibernation, a potentially reversible form of dysfunction. Cine imaging (without dobutamine stimulation) cannot differentiate between these possibilities.
Delayed Enhancement Magnetic Resonance Imaging
Similar to acute infarctions, chronic infarctions hyperenhance, whereas areas of hibernation do not. This finding allows differentiation between the two conditions, as discussed more fully later ( Fig. 28-3 ).
T2 Imaging
Chronic infarctions (>6 months’ duration), lacking edema, do not show increased signal on T2-weighted imaging, thus potentially allowing the distinction from acute MI. This distinction is often not possible with DE-MRI alone.
Clinical Reporting
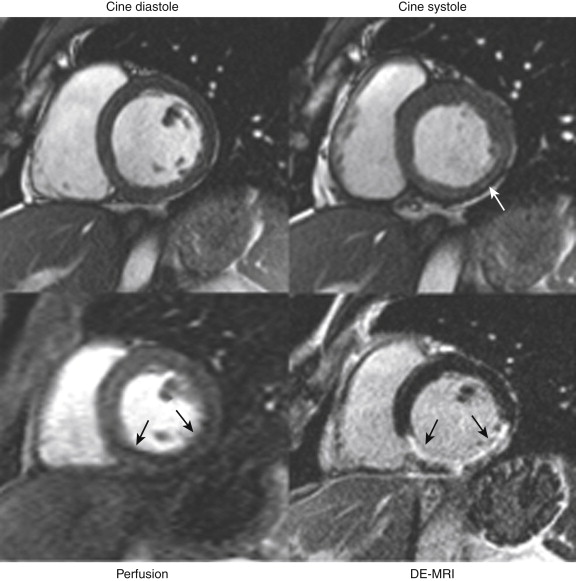
For general clinical reporting, the 17-segment model recommended by the American Heart Association (AHA) is used. This model divides the basal and midcavity levels into 6 segments each, an apical level into 4 segments, and the true apex into 1 segment (see Fig. 14-4 , A and B ). For each segment, left ventricular (LV) systolic function is graded visually using a 5-point scale ranging from normal wall thickening to systolic thinning and dyskinesis. The LV ejection fraction is also provided and is estimated from visual inspection of all the short- and long-axis views. Occasionally, LV ejection fraction is quantitatively measured by planimetry, such as in patients undergoing chemotherapy with potentially cardiotoxic agents. DE-MRI images are also interpreted using a 5-point scale. For each segment, the area or transmural extent of hyperenhanced tissue is graded visually. Examples of myocardial segments with various transmural extents of hyperenhancement are shown in Figure 28-4 .
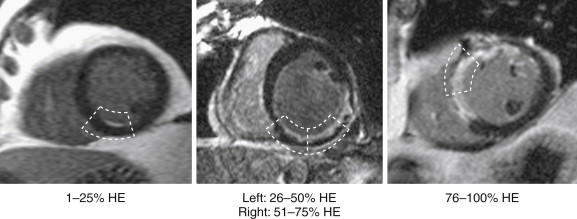
DE-MRI images must be interpreted with the immediately adjacent cine images. The cine images can provide a reference of the diastolic wall thickness of each region. This reference will be helpful if DE-MRI is performed before significant contrast washout from the LV cavity has occurred, and the examiner has difficulty in differentiating the bright signal from the LV cavity from hyperenhanced myocardium, as seen in Figure 28-5 .
Standard Examination Clinical Applications
Detection of Myocardial Infarction
In the usual clinical circumstance in which a patient presents with acute chest pain and testing shows elevated troponins as well as characteristic ECG changes (ST-segment elevation), the diagnosis of MI can be made with a high degree of certainty. However, many patients do not present during the acute phase and may not develop ECG changes diagnostic of earlier infarction. In these patients, significant myocardial injury may be missed. Troponin levels, although quite sensitive, may return to normal before the patient seeks medical attention. The ECG determination of infarction (postacute phase) is based on the development of Q waves, but any non–Q-wave infarction will therefore be missed. A universal definition of MI has been developed. It indicates that a new regional wall motion abnormality or a loss of viable myocardium can be considered evidence of earlier MI. However, wall motion abnormalities may not occur unless the infarcted region exceeds 20% to 50% of the myocardial wall thickness. Similarly, scintigraphic defects may not be apparent until more than 10 g of tissue is infarcted.
Thus, because a sizable threshold of damage is required, echocardiography or single photon emission computed tomography (SPECT) may miss MI, particularly when it is small or subendocardial. In these instances, in which the diagnosis of MI by the universal definition is difficult, DE-MRI may prove helpful. In fact, DE-MRI is the only imaging technique used for the detection of MI that has been validated by an international multicenter trial. In that study, as well as in other studies, DE-MRI was shown to detect even small areas of infarction, regardless of the duration of the infarct or reperfusion status.
The DE-MRI technique has been demonstrated to be the most sensitive means of imaging small areas of myocardial necrosis in vivo, and experimental studies demonstrated a nearly perfect spatial correlation between areas of hyperenhancement noted on MRI and areas of tissue necrosis on histologic slides ( Fig. 28-6 ). With standard imaging parameters, DE-MRI can detecting infarcts involving as little as one thousandth of total LV myocardial mass that may be undetectable by other techniques that assess myocardial perfusion or contractile function. DE-MRI is significantly more sensitive for the detection of infarction than is SPECT imaging. Although both modalities reliably detect transmural infarctions, MRI is superior for the detection of nontransmural subendocardial infarctions, more than 40% of which are missed by SPECT ( Fig. 28-7 ). MRI has also been shown to be superior to positron emission tomography (PET) scanning in the detection of subendocardial infarcts.


Detection of Unrecognized Myocardial Infarction: Populations and Prognosis
Because of its exquisite sensitivity, DE-MRI has also been used in population studies to evaluate the prevalence of unrecognized MI. DE-MRI has been found to be significantly more sensitive than ECG testing, with a significant increase in lesion detection ranging from 76% to 390%. One could postulate that the MIs not recognized by standard criteria are likely small and of uncertain significance. However, in studies of patients not clinically known to have had an MI, the presence of unrecognized MI by DE-MRI has been shown to confer a greater than sevenfold increased risk of major adverse cardiac events. The information from DE-MRI is a stronger predictor of outcomes than are the standard clinical risk factors and even catheterization data.
Non–ST-Segment Elevation Myocardial Infarction
The improved sensitivity and spatial localization of infarction provided by MRI may also allow its use in the evaluation of patients with non–ST-segment elevation MI. Specifically, in many of these patients who go on to subsequent cardiac catheterization, multivessel disease is present. The culprit artery responsible for the infarction may not be apparent from the catheter angiogram, particularly if intervening clot dissolution has occurred or if the affected vessel is occluded at its origin. In this instance, performance of DE-MRI before catheterization may allow definitive localization of the culprit artery ( Fig. 28-8 ).

Acute Chest Pain With Elevated Troponin: Myocardial Infarction or Not?
Other entities besides MI are known to cause acute chest pain syndromes, sometimes accompanied by troponin leak. Various studies evaluated the role of CMR in patients with chest pain, elevated troponins, and unobstructed coronary arteries. In this clinical setting, DE-MRI was reported to provide a new diagnosis in up to 65% of patients, and myocarditis was the most common identifiable cause. Similarly, in a large study of patients with ST-segment elevation MI (STEMI) who were undergoing coronary angiography, the investigators reported that 14% had no culprit artery detected, and 9.5% did not have significant CAD by invasive angiography. In the group without a clear culprit artery, MRI established that the most common diagnoses were myocarditis (31%), Takotsubo cardiomyopathy (31%), and STEMI without an angiographic lesion (28%) ( Fig. 28-9 ). These latter STEMIs may have been caused by coronary vasospasm, embolism, or occlusion with subsequent clot dissolution.

Potential causes responsible for acute coronary syndromes including MI in the absence of identifiable culprit artery include the absence of CAD, the absence of typical angiographic characteristics, or multivessel CAD in which more than one lesion is responsible.
Key Points
- 1.
Current standard diagnostic criteria (and SPECT imaging) may miss many MIs.
- 2.
The use of DE-MRI has been well validated as a means of detecting earlier infarction, and it may be useful in both population studies and prognostic studies.
- 3.
The use of DE-MRI to evaluate for MI in acute chest pain syndromes may allow distinction between patients with acute coronary syndromes and those with other entities such as myocarditis or Takotsubo cardiomyopathy.
- 4.
In those patients with MI and uncertainty regarding the culprit artery, MRI may provide additional helpful information.
Characterization of Myocardial Infarctions
Occasionally, circumstances arise in which the patient may have more than one MI, and the acuity of each is uncertain. As noted previously, DE-MRI demonstrates hyperenhancement in both acute and chronic MIs and thus cannot itself distinguish between these possibilities. However, findings suggesting that an area of hyperenhancement is likely chronic include wall thinning and lack of edema. Findings suggestive of acute MI include swelling of the affected segment, with edema noted as a bright signal abnormality on T2-weighted imaging.
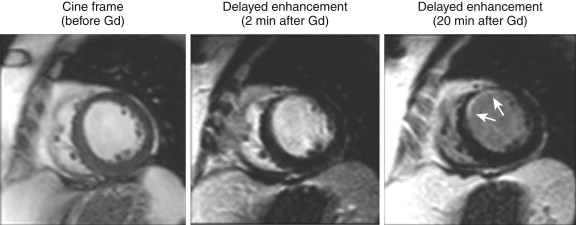
The presence of a dark, nonenhancing central area contained within an area of hyperenhancement on DE-MRI is termed a no-reflow zone and is also indicative of acute MI. This zone reflects an area of markedly delayed diffusion of contrast material into the central core of what is usually an extensive MI ( Fig. 28-10 ). The DE-MRI depiction of a no-reflow zone is thought to correspond to areas of profound microvascular obstruction indicative of an extensive ischemic injury resulting in microvascular destructive changes. These findings are often seen even after patency of the epicardial coronary artery has been restored. They are seen in some acute MIs (usually transmural in extent) and are transient phenomena, given that they are usually not visualized after 4 to 6 weeks. Within a given examination, they are also transient phenomena, more apparent at earlier time points and diminishing over time as contrast material diffuses into the area of injury. These areas should probably be properly termed slow-reflow zones ( Fig. 28-11 ). These areas of microvascular obstruction have been associated with worse outcomes, including adverse remodeling, an increased incidence of arrhythmia, and major adverse cardiac events and death.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree