Differential diagnosis of trigeminal neuralgia
Primary clinical characteristics
Mimicking characteristics
Glossopharyngeal neuralgia
Severe transient, stabbing or burning pain in the ear, base of the tongue, jaw, and tonsillar fossa
Pain in facial area; triggers can be chewing, swallowing, talking, or coughing
Geniculate neuralgia
Impairment of CN-VII sensory part; related to herpes zoster; pain is usually in the different ear structures
Pain may radiate from the ear to the face; has a burning dysesthetic quality
Herpetic and postherpetic neuralgia of the trigeminal nerve
Pain is steady and sustained, burning and aching. Often regresses in 2–3 weeks, months in patients older than 70 years of age
The steady pain is accompanied by shooting and sharp pain that radiates and is provoked by mechanical stimuli
Herpetic and postherpetic neuralgia of the cervical dorsal root ganglia
Steady pain in face, ear, and occiput
Facial pain, unilateral
Occipital neuralgia
Pain radiates following the greater occipital nerve distribution to the frontal region
Burning, unilateral pain that can radiate to the forehead, mimicking ophthalmic distribution of the trigeminal nerve
Atypical facial pain
Continuous aching or burning pain; unilateral or bilateral
Burning facial pain; may follow the nerve branches distribution; infrequently exacerbated by eating/talking
Rare disorders, that is, Raeder syndrome, trigeminal nerve neuritis from tumors, and other diseases
Usually Horner syndrome without anhidrosis
Sudden onset severe frontotemporal burning; often in periorbital or trigeminal distribution
Others: dental pathology, ear, nose, and throat; cluster or migraine headaches; temporomandibular joint syndrome
Variable presentations and patterns
Can mimic trigeminal neuralgia
The Gasserian Ganglion
Anatomy
Studying the gross and neuroanatomy of the trigeminal nerve is an essential task prior to neural blockade. The trigeminal nerve has both sensory and motor fibers. Visceral efferent fibers contribute to innervate some muscles of mastication and facial expression. Through its somatic afferent fibers, the trigeminal nerve transmits nociception, light touch, and temperature sensation from the skin of the face, teeth, anterior two thirds of the tongue, the nose, and oral cavity mucosa. Figure 11.1 shows the three branches of the trigeminal nerve (ophthalmic, maxillary, and mandibular).
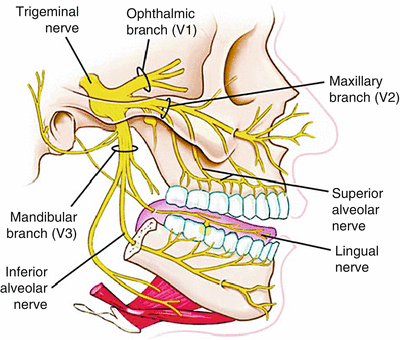

Fig. 11.1
The division of the three branches of the trigeminal nerve after exiting the middle cranial fossa (Copyright Elsevier)
The gasserian ganglion, also known as the trigeminal ganglion or the semilunar ganglion, sits in an invagination of the dura mater of the posterior cranial fossa, known as Meckel’s cave (Fig. 11.1). Injection of local anesthetic in Meckel’s cave, which contains cerebrospinal fluid, can potentially lead to total spinal anesthesia or spread to other cranial nerves. Its three sensory divisions, the ophthalmic (V1), maxillary (V2), and mandibular (V3), divide and exit anteriorly as shown in Fig. 11.1. The mandibular branch exiting through the foramen ovale has clinical applications as the reader will see in this chapter.
Blockade of the gasserian ganglion has been applied as surgical anesthetic for procedures of the head and neck in very limited instances. More commonly, it is used as a treatment for trigeminal neuralgia after failure of conservative therapy and also for cancer pain involving the face. Trigeminal ganglion neurolysis has been effective when oral therapy fails. The palliation of cancer-related pain arising from direct nerve involvement or surgical trauma has successfully been accomplished through blockade of the trigeminal ganglion or its divisions. Neurolysis of the trigeminal ganglion relieves cluster headaches refractory to oral therapy [8–12] and intractable atypical facial pain [13, 14].
Techniques for Gasserian Ganglion Blockade
To decrease the chances of adverse events, the use of radiological guidance such as computed tomography [15], fluoroscopy [16], or ultrasonography [17] along with a blunt-tipped curved needle is highly recommended. The blockade of the trigeminal ganglion has been performed without radiological guidance in the past but is not advisable. Those measures not only increase patient’s safety but also improve the access to the main anatomical landmark, the foramen ovale.
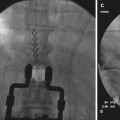
The patient is placed in a supine position with the head slightly extended. Facial skin is sterilely prepped. It is recommended that conscious sedation be administered for patient comfort with blood pressure, pulse oximetry, and electrocardiogram monitoring. Fluoroscopic x-ray guidance is used. The skin entry site is usually located approximately 2–3 cm lateral to the commissural labialis (the corner of the mouth) in a mid-pupillary line. Localization of the foramen ovale is critical to the success of this block. The anteroposterior fluoroscopic view usually shows the petrous ridge through the orbit, and 1 cm medially, it also shows a dip in the petrous ridge. Rotation of the C-arm head obliquely away from the nose approximately 20–30° and approximately 30–35° in the caudo-cranial direction will bring the foramen into view just medial to the mandible and at the top of the petrous “pyramid.” Lidocaine 1 % is applied to the skin and subcutaneous tissue over the shadow of the foramen ovale. For a diagnostic local anesthetic block, a 22-g B-bevel needle, 8–10 long, is used (for radiofrequency lesioning, an RFA 10-cm needle with a 2–5-mm active RFA tip is used). The needle is advanced through the entry point toward the foramen ovale, rotating the needle tip as needed to keep it on course initially downward and laterally, then medially aiming for the foramen ovale. To prevent intraoral entry, placement of one finger in the mouth could be done. When bone is encountered, the needle could be walked posteriorly along the skull into the foramen. A lateral view should be obtained, revealing the needle through the foramen ovale in a trajectory superior and toward the medial aspect of the external auditory meatus. A mandibular nerve paresthesia is commonly elicited. It is mandatory to test negative aspiration of cerebrospinal fluid (CSF) confirming that the needle did not penetrate the dura matter. The needle position is confirmed with injection of nonionic water-soluble contrast and negative aspiration of blood and CSF. For diagnostic local anesthetic blockade, small increments of local anesthetic (lidocaine 2 %, bupivacaine 0.5 %, or ropivacaine 0.5 %) are injected for a total of 1 ml. Monitoring the patient is essential to confirm that local anesthetic did not reach the CSF, putting the patient at risk of inadvertent spinal anesthetic and potential respiratory arrest. Figure 11.2 illustrates the trajectory of the needle in correct placement for the gasserian block.
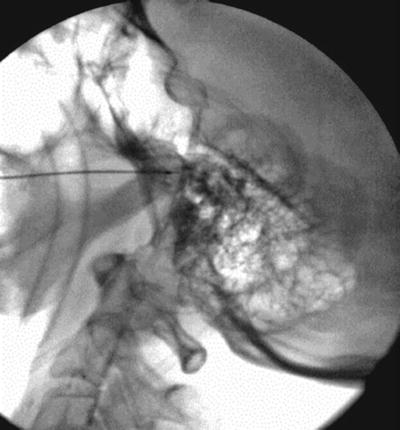

Fig. 11.2
Lateral fluoroscopic view showing trans-foramen ovale gasserian ganglion block: needle is in position and radio-opaque dye classic spread is shown
Chemical Neurolysis
Currently, the most common agent used for neurolysis of the trigeminal ganglion is glycerol [18–24], knowing that phenol [25] and alcohol [26–28] have also been used in the past. A neurolytic solution up to 0.5 ml should be injected in small increments preferably of 0.1 ml to avoid inadvertent spread to structures of the brain stem. For the technique using glycerol, the needle is advanced into the trigeminal cistern and free CSF flow is confirmed. When using a hyperbaric neurolytic agent, the patient should sit with the head tipped forward for 2 h [29]. This maneuver ensures spread of the injectate to the maxillary and mandibular branches, sparing the ophthalmic branch. Acute unilateral total visual loss after gasserian phenol injection has been reported [30].
Radiofrequency Ablation
Conventional radiofrequency (RF) neurolysis is performed at temperatures ranging from 60° to 90° centigrade, for duration of 60–90 s. Knowing that the mandibular branch is the only branch carrying motor fibers, motor stimulation at 2 Hz within a range of 0.1–1.5 V will reproduce muscle contraction of the lower mandible. While performing lesioning for V1 or V2, motor stimulation at 2 Hz is not expected to show any muscular contraction. For confirmation of needle position, sensory stimulation at 50 Hz preferably below 0.6 V should precede any treatment. A correct needle position should reproduce a tingling-like sensation or paresthesia in the distribution of the targeted nerve branch. Adjustment of the needle position is performed to optimize desirable sensory patterns prior to any lesioning. Patient alertness and cooperation is of paramount importance: Patient feedback on where the sensation is elicited will help the physician complete the desired block successfully (Fig. 11.2). Understanding the anatomy and how the rootlets of the trigeminal ganglion lay in a superomedial to inferolateral plane is very important: In case of a non-desirable motor response, the practitioner will adjust the needle from a lateral position to a more medial one. Confirmation of negative blood flow should be documented. Up to 0.5 ml of 0.5 % bupivacaine or 0.2 % ropivacaine should be injected prior to RF lesioning to alleviate procedure-related discomfort. If RF lesioning is performed on the 1st branch of the trigeminal nerve (V1), temperature should be limited to 60° to preserve the corneal reflex.
The Trigeminal Nerve Branches: Opthalmic, Maxillary, and Mandibular
Anatomy
The ophthalmic branch (V1) of the trigeminal branch is a purely sensory branch [36]. It enters the orbit via the superior orbital fissure. In turn, it divides into three branches, the frontal, nasociliary, and lacrimal nerves. The latter two provide innervations to nasal structures and the lacrimal gland, respectively. The supraorbital and the supratrochlear are terminal branches of the frontal nerve: They exit the orbit anteriorly and provide innervations to upper eyelid, forehead, and anterior scalp. As illustrated in the next paragraph, they are clinically most significant of the V1 branches.
The maxillary branch (V2) is also a pure sensory branch [37]. It exits the middle cranial fossa into the pterygopalatine fossa in a horizontal fashion through the foramen rotundum. Then, it passes through the inferior orbital fissure to the orbit before exiting to the face via the infraorbital foramen. That passage through the four facial compartments lead to the division of the many branches of V2 to four regional groups of branches. Understanding the exit of V2 from the middle cranial fossa to the face simplifies the understanding of its innervations of facial structures (Fig. 11.1). Table 11.2 summarizes the four groups and the facial areas they innervate.
Table 11.2
The four regional groups of V2
The four regional groups of V2 | V2 nerve branches | Facial areas innervated |
|---|---|---|
1. Intracranial group | Middle meningeal nerve | The dura matter of the middle cranial fossa |
2. Pterygopalatine group | Zygomatic nerve | The temporal and zygomatic region |
The sphenopalatine branches | The mucosa of the maxillary sinus, upper molars, upper gums, and the mucous membrane of the cheek | |
3. Infraorbital canal group | Anterosuperior alveolar branch | Incisors, canines, anterior wall of the maxillary antrum, floor of nasal cavity |
Middle superior branch | Premolars | |
4. Infraorbital facial group | Inferior palpebral branch | Conjunctiva and skin of lower eyelid |
External nasal branch | Side of the nose | |
Superior labial branch | Skin of the upper lip and part of the oral mucosa |
The mandibular nerve is formed by the joining of the large sensory mandibular division of the trigeminal nerve and a small motor nerve root. They both cross the foramen ovale leaving the middle cranial fossa and forming the mandibular nerve [37]. This combined trunk then divides into a small anterior and larger posterior trunk (Fig. 11.1). Prior to this division, it gives off the nervus spinosus, innervating the dura matter and mucosal lining of the mastoid sinus, and the internal pterygoid, innervating the internal pterygoid and sending branches to the otic ganglion.
From the anterior trunk comes the buccinator nerve to innervate the skin and mucous membrane overlying the buccinator muscle. The anterior trunk also gives off three motor branches: the masseteric, deep temporal nerves, and the external pterygoid nerve. They provide motor innervations to the masseter muscle, temporalis muscle, and external pterygoid muscle, respectively.
The posterior trunk contains mostly sensory fibers. The following branches come off the posterior trunk: The auriculotemporal nerve provides sensory innervations to the following structures – the tympanic membrane, the lining of the acoustic meatus, the posterior temporomandibular joint, the parotid gland, the skin overlying the temporal region, and the skin anterior to the tragus and helix. The lingual nerve innervates the dorsum and lateral aspects of the anterior 2/3 of the tongue, the lateral mucous membrane, and the sublingual gland. The inferior alveolar nerve innervates the lower teeth and the mandible. Its terminal branch, the mental nerve, innervates the chin and the skin and mucous membrane of the lower lip.
Blockade of the Ophthalmic Branch
The most commonly blocked branches of V1 are the supraorbital and supratrochlear branches. To block the supraorbital nerve, the patient is placed in a supine position. The landmark for this block, the supraorbital foramen, is palpated along the upper border of the orbit. After skin prepping, with precautions not to spill any disinfecting solution in the eye, a 25-gauge, 1.5-in.-long needle is introduced into the skin through the identified supraorbital foramen in a perpendicular plane to the skin. Some paresthesia is usually elicited; then, 3–4 ml of local anesthetic solution (lidocaine 1–2 %, bupivacaine 0.5 %, or ropivacaine 0.2 %) is injected in a fanlike fashion. Radiofrequency lesioning for the supraorbital nerve as a treatment for postherpetic neuralgia has been described. The technique for radiofrequency lesioning is similar to the local anesthetic block with the exception of using a RFA needle and confirmation of positive sensory response at 2-Hz stimulation in the somatic nerve distribution. To achieve a blockade of the supratrochlear branch of the ophthalmic division, simply direct the needle medially at the level of the supraorbital foramen and repeat similar steps as described above.
Blockade of the Maxillary and Mandibular Branches
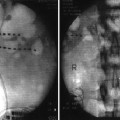
The preferred approach for blockade of the maxillary (V2) and the mandibular (V3) branches of the trigeminal nerve is through the mandibular notch, also known as the coronoid notch. These blocks could be done without radiologic guidance. However, fluoroscopic guidance is highly recommended. Patient is placed in supine position, and the x-ray C arm is placed in a lateral view. The patient is asked to open and close his/her mouth few times if possible to facilitate palpation of the mandibular notch which could be marked. After skin sterilization, the skin is anesthetized with lidocaine 1 %. A 22-g B-bevel needle, 8–10 cm long is used (for radiofrequency lesioning, an RFA needle, 10 cm long, with a 2–5-mm active RFA tip is used). The needle is introduced under fluoroscopic guidance at the site already marked and advanced in a horizontal plane. Fluoroscopic guidance is used to direct the needle tip through the infratemporal fossa (Fig. 11.3). A small-angulation cephalad and slightly posterior will allow the needle to be in proximity to the lateral nasal mucosa taking extreme care not to pierce through it. The end point of the advancement of the needle is the lateral pterygoid plate. If the maxillary nerve is the final target, a slight superior and posterior angulation will elicit paresthesia into this nerve distribution (nose ridge, upper lip, gum, and face). If the mandibular nerve is targeted, a slight caudad and anterior angulation usually elicits paresthesia in its somatic distribution (lower mandible, lower lip, lower jaw, and tongue). If radiofrequency lesioning is planned, sensory testing at 50 Hz should be achieved preferably below 0.6 V in the nerve distribution prior to any treatment. Negative aspiration of blood and CSF should be demonstrated prior to any treatment. Figure 11.4 shows the advancement and final position of the needle. Figure 11.5 shows RF needle in position.
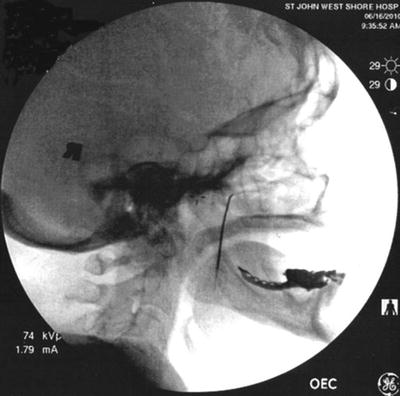
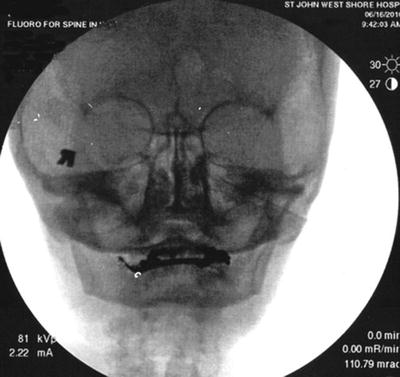
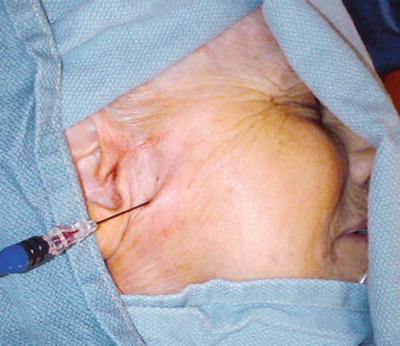

Fig. 11.3
Lateral fluoroscopic view showing needle though the coronoid notch in position for advancement

Fig. 11.4
Anteroposterior fluoroscopic view showing needle advancement for maxillary nerve block

Fig. 11.5
Final needle placement for radiofrequency lesioning of the maxillary nerve
Efficacy and Safety
The efficacy and safety of radiofrequency (RF) lesioning for trigeminal neuralgia have been described in the literature. Review of current literature reveals one retrospective uncontrolled chart review [38] and four prospective uncontrolled clinical trials [39–42]. No randomized sham-controlled trials on the value of RF treatment of the trigeminal ganglion have been published. An initial complete pain relief was reported in 83–99 % of patients treated with RF ablation in some studies [39, 40, 43–49]. Repeating the procedure has increased the long-term efficacy in three studies [38, 40, 42]. Kanpolat et al. [38] reviewed the records of 1,600 patients that received percutaneous radiofrequency rhizotomy as a treatment for trigeminal neuralgia. Even though initially up to 97.6 % reported acute pain relief at 5-year follow-up, only 57.7 % reported complete pain relief with a single procedure. This figure reaches 94.2 % for patients receiving multiple procedures. At 10- and 20-year follow-up, the percentage for single procedure was 52.3 % and 41 %, respectively, and for multiple procedures was 94.2 % and 100 %, respectively. The authors concluded that this technique is safe and effective.
Long-term safety from prospective uncontrolled and retrospective clinical studies up to 20 years was demonstrated. Taha and Tew [50] conducted a 15-year prospective study following 154 patients with trigeminal neuralgia treated with percutaneous stereotactic radiofrequency rhizotomy. Initial success was reported at 99 % after a single treatment. Similar results were confirmed in a prospective study by Scrivani et al. following 215 patients with trigeminal neuralgia for 15 years following rhizotomy. They found that patients had pain relief in 92 %. Table 11.3 summarizes the efficacy of RF lesioning for facial pain, trigeminal neuralgia, and headache.
Table 11.3
Results of radiofrequency lesioning studies
Study | Technique | Results (%) | Number of patients | Comments |
|---|---|---|---|---|
Kanpolat et al. [38] | RF | 41–100 | 1,600 | TN, 20-year follow-up |
Taha et al. [39] | RF | 99 | 154 | TN, 15-year follow-up |
Zakrzewska et al. [41] | RF | 36–40 months pain-free | 48 | Chronic facial pain |
Onofrio [43] | RF | 86 | 140 | Mainly TN |